FWP: Filing under Securities Act Rules 163/433 of free writing prospectuses
Published on March 20, 2025
|
|
Issuer Free Writing Prospectus dated March 20, 2025 Filed Pursuant to Rule 433 Relating to Preliminary Prospectus Supplement dated March 20, 2025 Registration No. 333-276462 |

Delivering on the Promise of Gene Therapy for Rare Inherited Retinal
Diseases Braydon, RDH12 patient
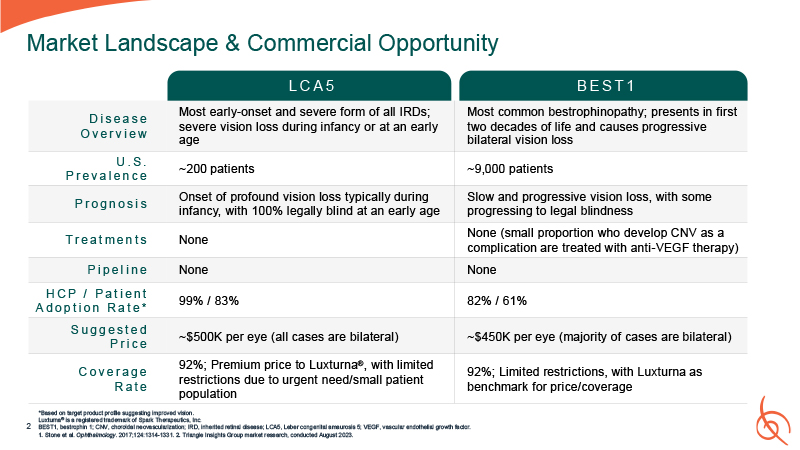
Market Landscape & Commercial Opportunity D i s e a s e O v e r v i e w Most
early-onset and severe form of all IRDs; severe vision loss during infancy or at an early age Most common bestrophinopathy; presents in first two decades of life and causes progressive bilateral vision loss U . S . P r e v a l e n c e ~200
patients ~9,000 patients P r o g n o s i s Onset of profound vision loss typically during infancy, with 100% legally blind at an early age Slow and progressive vision loss, with some progressing to legal blindness T r e a t m e n t
s None None (small proportion who develop CNV as a complication are treated with anti-VEGF therapy) P i p e l i n e None None H C P / P a t i e n t A d o p t i o n R a t e * 99% / 83% 82% / 61% S u g g e s t e d P r i c e ~$500K per
eye (all cases are bilateral) ~$450K per eye (majority of cases are bilateral) C o v e r a g e R a t e 92%; Premium price to Luxturna®, with limited restrictions due to urgent need/small patient population 92%; Limited restrictions, with
Luxturna as benchmark for price/coverage L C A 5 B E S T 1 Luxturna® is a re gistere d trad emark of Spark Th erapeu tics, Inc. *Based on ta rget p roduct pr ofile sugge sting improved vision . 2 BES T1, bestrophin 1; CNV, choroida l ne ova
scularization; IRD, inhe rited retinal disease; LCA5 , Lebe r cong enital ama urosis 5; VEGF, vascu lar endothelial gro wth factor. 1. Stone et al. Op hth almology. 201 7;1 24:1314-133 1. 2. Triangle Insights Gr oup market r ese arch, conducted
August 2 023.
One-year Results from a Phase 1/2 Study of OPGx-LCA5 Gene Therapy for
LCA5 Alan, LCA5 patient LCA5, Leber congenital amaurosis 5.
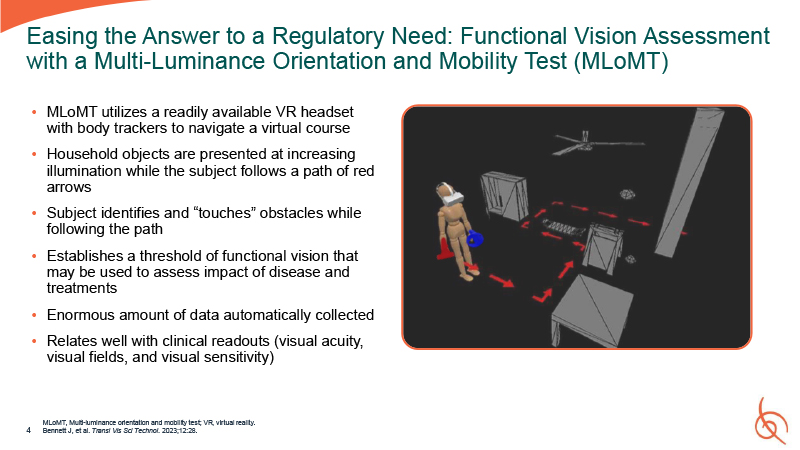
MLoMT utilizes a readily available VR headset with body trackers to navigate a
virtual course Household objects are presented at increasing illumination while the subject follows a path of red arrows Subject identifies and “touches” obstacles while following the path Establishes a threshold of functional vision that may
be used to assess impact of disease and treatments Enormous amount of data automatically collected Relates well with clinical readouts (visual acuity, visual fields, and visual sensitivity) MLoMT, Multi-luminance orientation and mobility test;
VR, virtual reality. 4 Bennett J, et al. Transl Vis Sci Technol. 2023;12:28. Easing the Answer to a Regulatory Need: Functional Vision Assessment with a Multi-Luminance Orientation and Mobility Test (MLoMT)
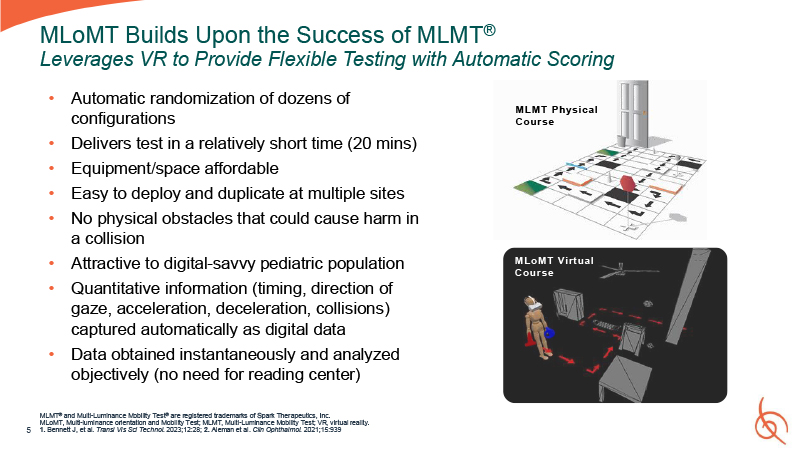
5 MLoMT Builds Upon the Success of MLMT® Leverages VR to Provide Flexible Testing
with Automatic Scoring MLMT® and Multi-Luminance Mobility Test® are registered trademarks of Spark Therapeutics, Inc. MLoMT, Multi-luminance orientation and Mobility Test; MLMT, Multi-Luminance Mobility Test; VR, virtual reality. 1. Bennett J,
et al. Transl Vis Sci Technol. 2023;12:28; 2. Aleman et al. Clin Ophthalmol. 2021;15:939 Automatic randomization of dozens of configurations Delivers test in a relatively short time (20 mins) Equipment/space affordable Easy to deploy and
duplicate at multiple sites No physical obstacles that could cause harm in a collision Attractive to digital-savvy pediatric population Quantitative information (timing, direction of gaze, acceleration, deceleration, collisions) captured
automatically as digital data Data obtained instantaneously and analyzed objectively (no need for reading center) MLMT Physical Course MLoMT Virtual Course
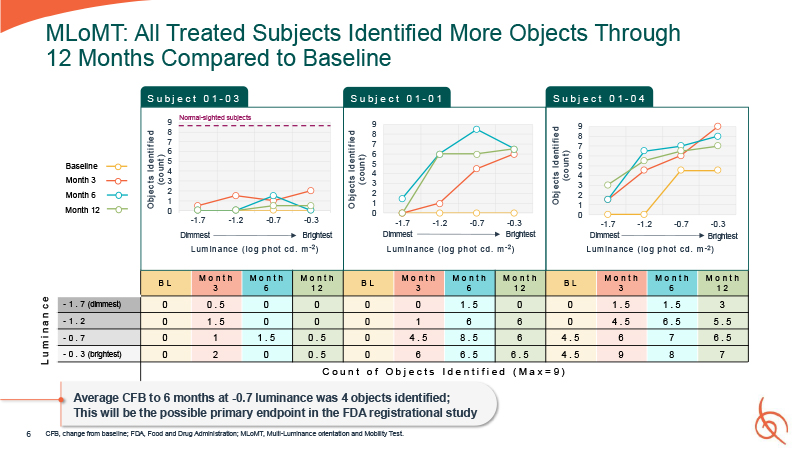
6 MLoMT: All Treated Subjects Identified More Objects Through 12 Months Compared to
Baseline L u m i n a n c e CFB, change from baseline; FDA, Food and Drug Administration; MLoMT, Multi-Luminance orientation and Mobility Test. S u b j e c t 0 1 - 03 O bj ects Id e nti f ie d ( co un t ) S u b j e c t 0 1 - 01 O bj
ects Id e nti f ie d ( co un t ) O bj ects Id e nti f ie d ( co un t ) S u b j e c t 0 1 - 04 Baseline Month 3 Month 6 Month 12 9 Normal-sighted
subjects 9 8 7 6 5 4 3 2 8 7 6 5 4 3 2 1 0 9 8 7 6 5 4 3 2 1 1 0 -1.7 -1.2 -0.7 -0.3 Dimmest Brightest L um in a nce ( lo g p h ot cd . m - 2 ) -1.7 -1.2 -0.7 -0.3 Dimmest Brightest L um in a nce ( lo g p h ot cd
. m - 2 ) 0 -1.7 -1.2 -0.7 -0.3 Dimmest Brightest L um in a nce ( lo g p h ot cd . m - 2 ) B L M o n t h 3 M o n t h 6 M o n t h 1 2 B L M o n t h 3 M o n t h 6 M o n t h 1 2 B L M o n t h 3 M o n t h 6 M o n t h 1 2
- 1 . 7 (dimmest) 0 0 . 5 0 0 0 0 1 . 5 0 0 1 . 5 1 . 5 3 - 1 . 2 0 1 . 5 0 0 0 1 6 6 0 4 . 5 6 . 5 5 . 5 - 0 . 7 0 1 1 . 5 0 . 5 0 4 . 5 8 . 5 6 4 . 5 6 7 6 . 5 - 0 . 3 (brightest) 0 2 0 0 .
5 0 6 6 . 5 6 . 5 4 . 5 9 8 7 C o u n t o f O b j e c t s I d e n t i f i e d ( M a x = 9 ) Average CFB to 6 months at -0.7 luminance was 4 objects identified; This will be the possible primary endpoint in the FDA registrational study
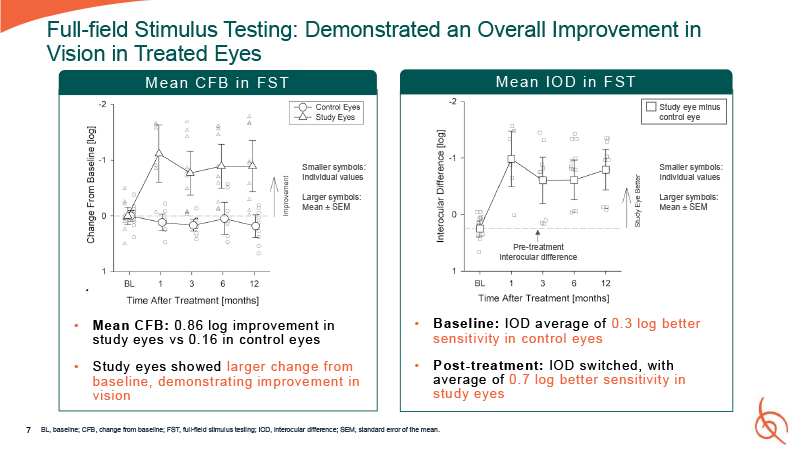
7 Full-field Stimulus Testing: Demonstrated an Overall Improvement in Vision in
Treated Eyes Mean CFB: 0. 86 log improvement in study eyes vs 0. 16 in control eyes Study eyes showed larger change from baseline, demonstrating improvement in vision Pre-treatment interocular difference Baseline: IOD average of 0. 3 log
better sensitivity in control eyes Post-treatment: IOD switched, with average of 0. 7 log better sensitivity in study eyes BL, baseline; CFB, change from baseline; FST, full-field stimulus testing; IOD, interocular difference; SEM, standard
error of the mean. Study eye minus control eye Smaller symbols: Individual values Larger symbols: Mean ± SEM Smaller symbols: Individual values Larger symbols: Mean ± SEM Mean CFB in FST Mean IO D in F ST
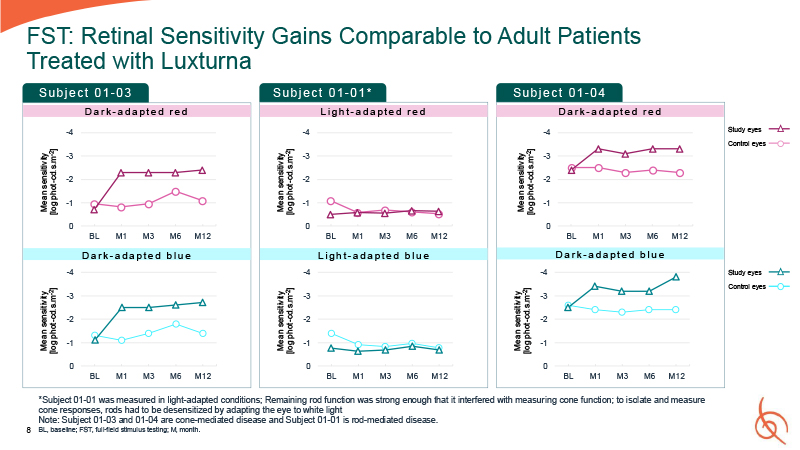
8 FST: Retinal Sensitivity Gains Comparable to Adult Patients Treated with
Luxturna -4 -3 -2 -1 0 BL M1 M3 M6 M12 Mean sensitivity [log phot-cd.s.m-2] -4 -3 -2 -1 0 Mean sensitivity [log phot-cd.s.m-2] -4 -3 -2 -1 0 BL M1 M3 M6 M12 Mean sensitivity [log phot-cd.s.m-2] -4 -3 -2 -1 0 Mean
sensitivity [log phot-cd.s.m-2] -4 -3 -2 -1 0 BL M1 M3 M6 M12 Mean sensitivity [log phot-cd.s.m-2] -4 -3 -2 -1 0 Mean sensitivity [log phot-cd.s.m-2] Su b j e c t 0 1 - 03 Su b j e c t 0 1 - 01 * Su b j e c t 0 1 - 04 D a r k -
a d a p t e d r e d L i g h t - a d a p t e d r e d D a r k - a d a p t e d r e d BL M1 M3 M6 M12 BL M1 M3 M6 M12 BL M1 M3 M6 M12 *Subject 01-01 was measured in light-adapted conditions; Remaining rod function was strong enough that it
interfered with measuring cone function; to isolate and measure cone responses, rods had to be desensitized by adapting the eye to white light Note: Subject 01-03 and 01-04 are cone-mediated disease and Subject 01-01 is rod-mediated
disease. BL, baseline; FST, full-field stimulus testing; M, month. D a r k - a d a p t e d b l u e L i g h t - a d a p t e d b l u e D a r k - a d a p t e d b l u e Study eyes Control eyes Study eyes Control eyes
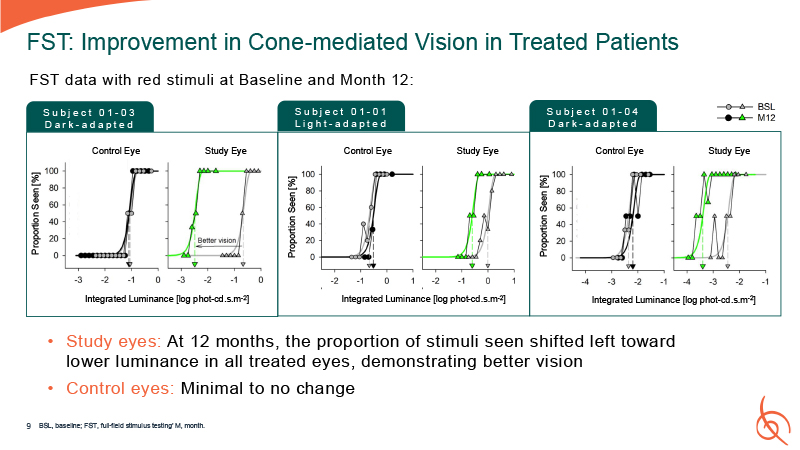
FST: Improvement in Cone-mediated Vision in Treated Patients Control Eye Study
Eye S u b j e c t 0 1 - 03 D a r k - a d a p t e d Proportion Seen [%] Integrated Luminance [log phot-cd.s.m-2] Control Eye Study Eye Proportion Seen [%] Integrated Luminance [log phot-cd.s.m-2] Control Eye Study Eye Proportion Seen
[%] Integrated Luminance [log phot-cd.s.m-2] S u b j e c t 0 1 - 01 L i g h t - a d a p t e d S u b j e c t 0 1 - 04 D a r k - a d a p t e d FST data with red stimuli at Baseline and Month 12: Study eyes: At 12 months, the proportion of
stimuli seen shifted left toward lower luminance in all treated eyes, demonstrating better vision Control eyes: Minimal to no change BSL, baseline; FST, full-field stimulus testing’ M, month. 9
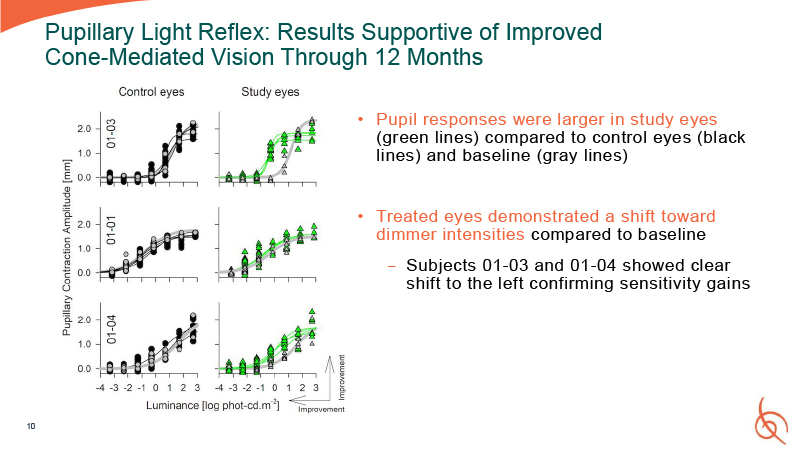
Pupillary Light Reflex: Results Supportive of Improved Cone-Mediated Vision Through
12 Months Pupil responses were larger in study eyes (green lines) compared to control eyes (black lines) and baseline (gray lines) Treated eyes demonstrated a shift toward dimmer intensities compared to baseline − Subjects 01-03 and 01-04
showed clear shift to the left confirming sensitivity gains 10
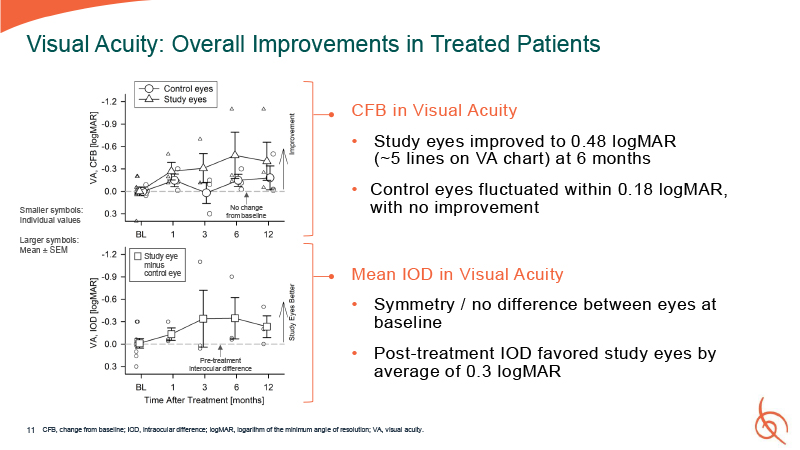
11 Visual Acuity: Overall Improvements in Treated Patients CFB in Visual
Acuity Study eyes improved to 0.48 logMAR (~5 lines on VA chart) at 6 months Control eyes fluctuated within 0.18 logMAR, with no improvement Mean IOD in Visual Acuity Symmetry / no difference between eyes at baseline Post-treatment IOD
favored study eyes by average of 0.3 logMAR CFB, change from baseline; IOD, intraocular difference; logMAR, logarithm of the minimum angle of resolution; VA, visual acuity. Pre-treatment interocular difference No change from baseline Study
eye minus control eye Smaller symbols: Individual values Larger symbols: Mean ± SEM
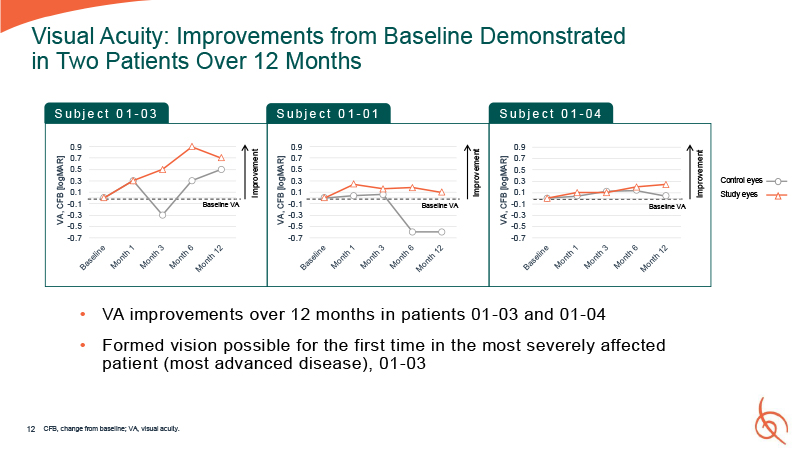
0.9 0.7 0.5 0.3 0.1 -0.1 -0.3 -0.5 -0.7 VA, CFB
[logMAR] 0.9 0.7 0.5 0.3 0.1 -0.1 -0.3 -0.5 -0.7 VA, CFB [logMAR] 12 Visual Acuity: Improvements from Baseline Demonstrated in Two Patients Over 12 Months 0.9 0.7 0.5 0.3 0.1 -0.1 -0.3 -0.5 -0.7 VA, CFB [logMAR] Control
eyes Study eyes S u b j e c t 0 1 - 03 S u b j e c t 0 1 - 01 S u b j e c t 0 1 - 04 Improvement VA improvements over 12 months in patients 01 -03 and 01-04 Formed vision possible for the first time in the most severely affected
patient (most advanced disease), 01-03 CFB, change from baseline; VA, visual acuity. Improvement Improvement Baseline VA Baseline VA Baseline VA
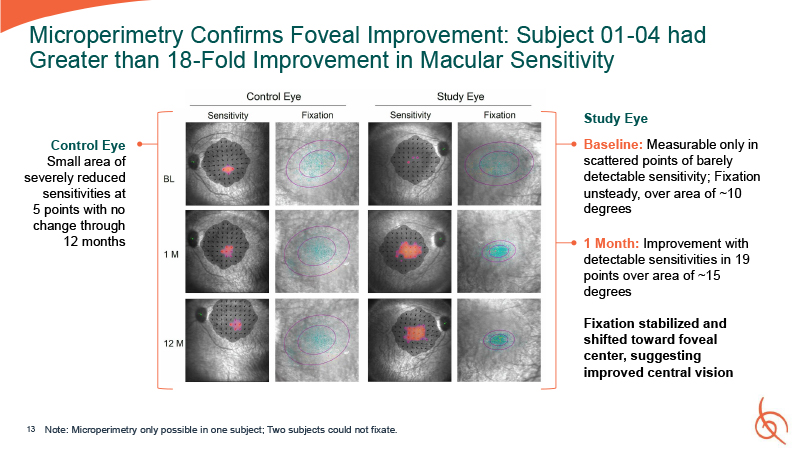
Microperimetry Confirms Foveal Improvement: Subject 01-04 had Greater than 18-Fold
Improvement in Macular Sensitivity Study Eye Baseline: Measurable only in scattered points of barely detectable sensitivity; Fixation unsteady, over area of ~10 degrees 1 Month: Improvement with detectable sensitivities in 19 points over area
of ~15 degrees Fixation stabilized and shifted toward foveal center, suggesting improved central vision 13 Note: Microperimetry only possible in one subject; Two subjects could not fixate. Control Eye Small area of severely reduced
sensitivities at 5 points with no change through 12 months
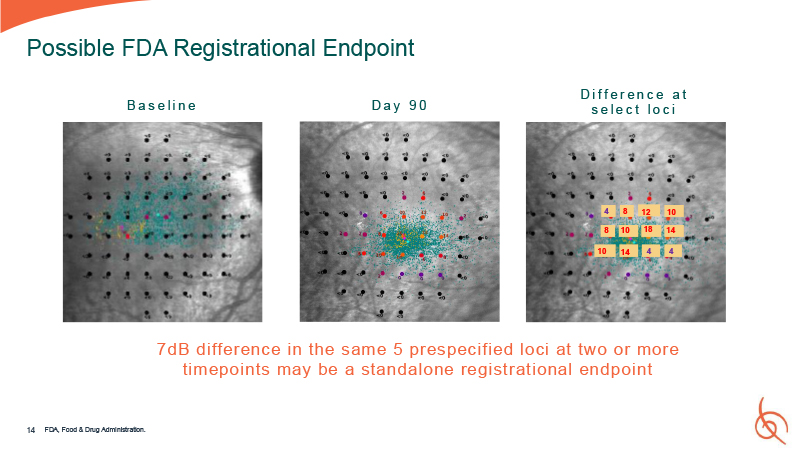
B a s e l i n e D a y 9 0 4 4 4 8 12 10 14 18 8 10 14 10 14 Possible
FDA Registrational Endpoint 7dB difference in the same 5 prespecified loci at two or more timepoints may be a standalone registrational endpoint D i f f e r e n c e a t s e l e c t l o c i FDA, Food & Drug Administration.
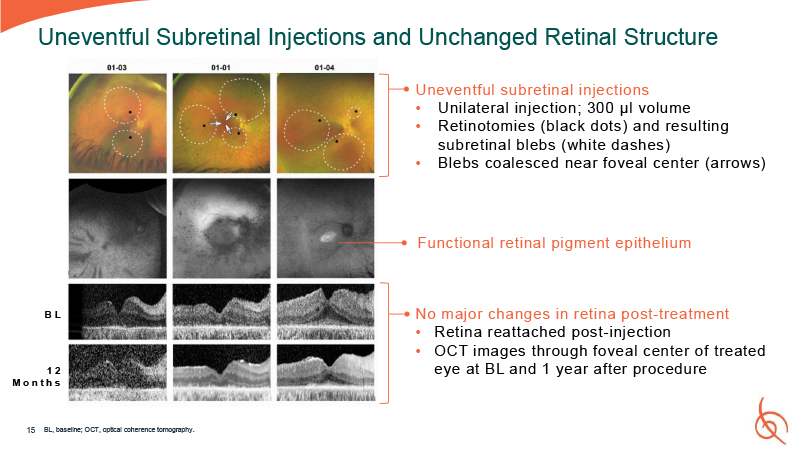
15 Uneventful Subretinal Injections and Unchanged Retinal Structure Uneventful
subretinal injections Unilateral injection; 300 µl volume Retinotomies (black dots) and resulting subretinal blebs (white dashes) Blebs coalesced near foveal center (arrows) No major changes in retina post-treatment Retina reattached
post-injection OCT images through foveal center of treated eye at BL and 1 year after procedure B L 12 M o n t h s Functional retinal pigment epithelium BL, baseline; OCT, optical coherence tomography.

No dose-limiting toxicities No serious adverse events Anticipated adverse events
were mild and unrelated to treatment − Mostly related to use of systemic steroid or with surgical procedure All early AEs resolved within 30 days of the procedure 16 OPGx-LCA5 was Safe and Well-Tolerated Through One Year AE, adverse event;
LCA5, Leber congenital amaurosis 5.
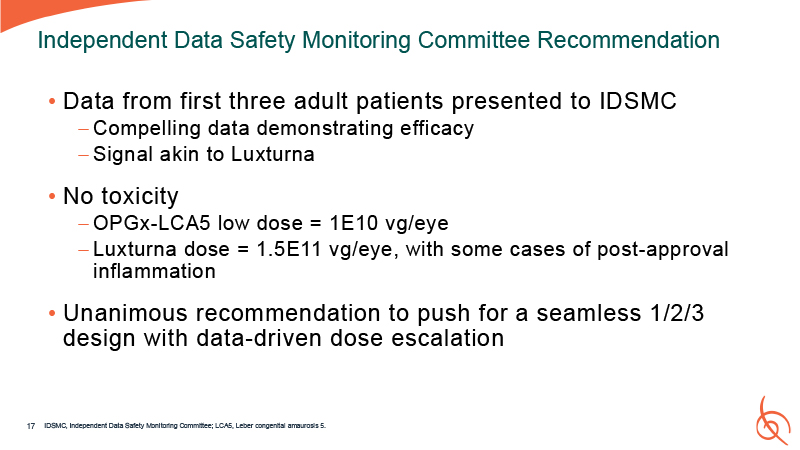
Data from first three adult patients presented to IDSMC Compelling data
demonstrating efficacy Signal akin to Luxturna No toxicity OPGx-LCA5 low dose = 1E10 vg/eye Luxturna dose = 1.5E11 vg/eye, with some cases of post-approval inflammation Unanimous recommendation to push for a seamless 1/2/3 design with
data-driven dose escalation Independent Data Safety Monitoring Committee Recommendation IDSMC, Independent Data Safety Monitoring Committee; LCA5, Leber congenital amaurosis 5. 17

“I initially agreed to participate in trial to help people, including a child in our
own neighborhood who also has LCA5. I wanted this research to advance understanding and treatments that could make a difference for him and so many others. After surgery and recovery, I’ve found the trial and treatment has also helped me find
more joy in the little things—now I'm able to see fireworks light up the sky and appreciate the food on my plate—and improve my ability to do daily tasks that those without retinal diseases may take for granted like spotting my Uber and using
utensils. I look forward to the possibility of treating my other eye once the trial advances to Phase 2.” - OPGx-LCA5 Clinical Trial Participant A Patient’s Journey in the OPGx-LCA5 Trial: Helping Others and Life-changing Moments “ “ LCA5,
Leber congenital amaurosis 5. 18

OPGx-LCA5 Clinical Development Plan Alan, LCA5 patient LCA5, Leber congenital
amaurosis 5.
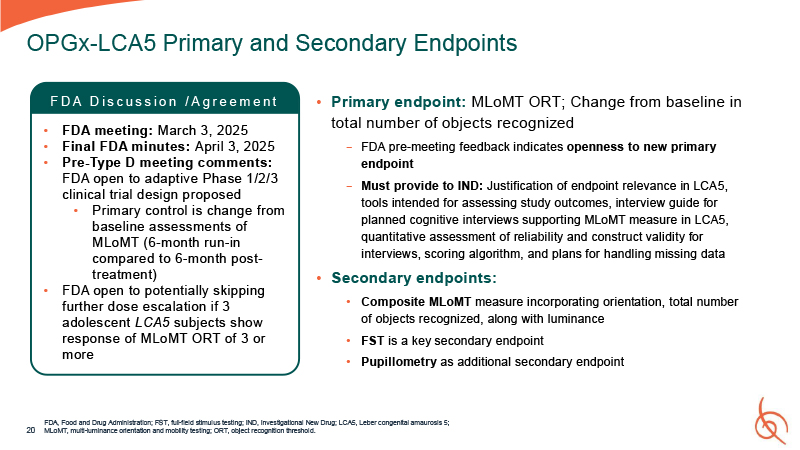
Primary endpoint: MLoMT ORT; Change from baseline in total number of objects
recognized − FDA pre-meeting feedback indicates openness to new primary endpoint − Must provide to IND: Justification of endpoint relevance in LCA5, tools intended for assessing study outcomes, interview guide for planned cognitive interviews
supporting MLoMT measure in LCA5, quantitative assessment of reliability and construct validity for interviews, scoring algorithm, and plans for handling missing data Secondary endpoints: Composite MLoMT measure incorporating orientation, total
number of objects recognized, along with luminance FST is a key secondary endpoint Pupillometry as additional secondary endpoint FDA, Food and Drug Administration; FST, full-field stimulus testing; IND, Investigational New Drug; LCA5, Leber
congenital amaurosis 5; 20 MLoMT, multi-luminance orientation and mobility testing; ORT, object recognition threshold. OPGx-LCA5 Primary and Secondary Endpoints F D A D i s c u s s i o n / A g r e e m e n t FDA meeting: March 3, 2025 Final
FDA minutes: April 3, 2025 Pre-Type D meeting comments: FDA open to adaptive Phase 1/2/3 clinical trial design proposed Primary control is change from baseline assessments of MLoMT (6-month run-in compared to 6-month post- treatment) FDA
open to potentially skipping further dose escalation if 3 adolescent LCA5 subjects show response of MLoMT ORT of 3 or more
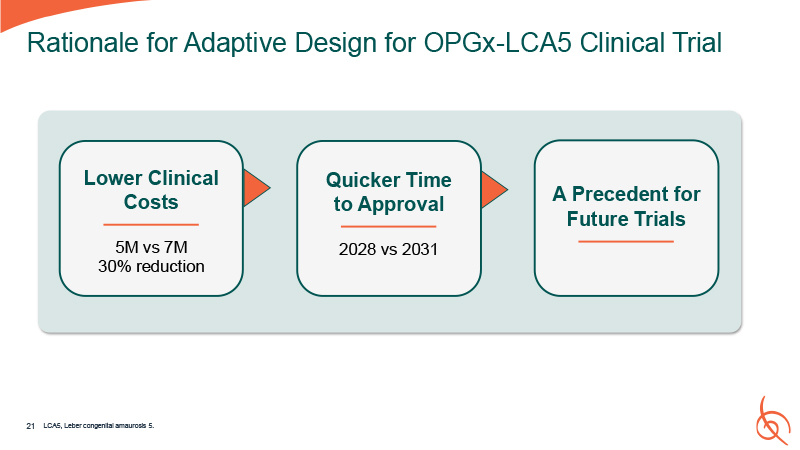
A Precedent for Future Trials Quicker Time to Approval 2028 vs 2031 Rationale for
Adaptive Design for OPGx-LCA5 Clinical Trial Lower Clinical Costs 5M vs 7M 30% reduction 21 LCA5, Leber congenital amaurosis 5.
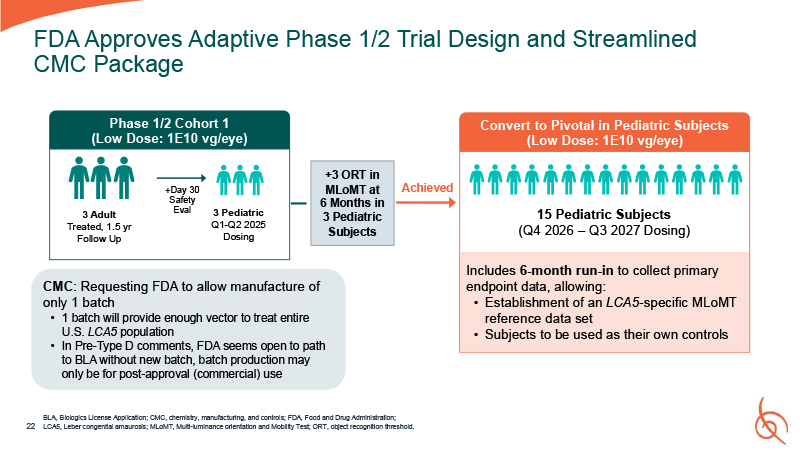
FDA Approves Adaptive Phase 1/2 Trial Design and Streamlined CMC Package BLA,
Biologics License Application; CMC, chemistry, manufacturing, and controls; FDA, Food and Drug Administration; 22 LCA5, Leber congenital amaurosis; MLoMT, Multi-luminance orientation and Mobility Test; ORT, object recognition threshold. 3
Pediatric Q1-Q2 2025 Dosing 3 Adult Treated, 1.5 yr Follow Up Phase 1/2 Cohort 1 (Low Dose: 1E10 vg/eye) +Day 30 Safety Eval +3 ORT in MLoMT at 6 Months in 3 Pediatric Subjects Convert to Pivotal in Pediatric Subjects (Low Dose: 1E10
vg/eye) 15 Pediatric Subjects (Q4 2026 – Q3 2027 Dosing) Achieved Includes 6-month run-in to collect primary endpoint data, allowing: Establishment of an LCA5-specific MLoMT reference data set Subjects to be used as their own
controls CMC: Requesting FDA to allow manufacture of only 1 batch 1 batch will provide enough vector to treat entire U.S. LCA5 population In Pre-Type D comments, FDA seems open to path to BLA without new batch, batch production may only be
for post-approval (commercial) use
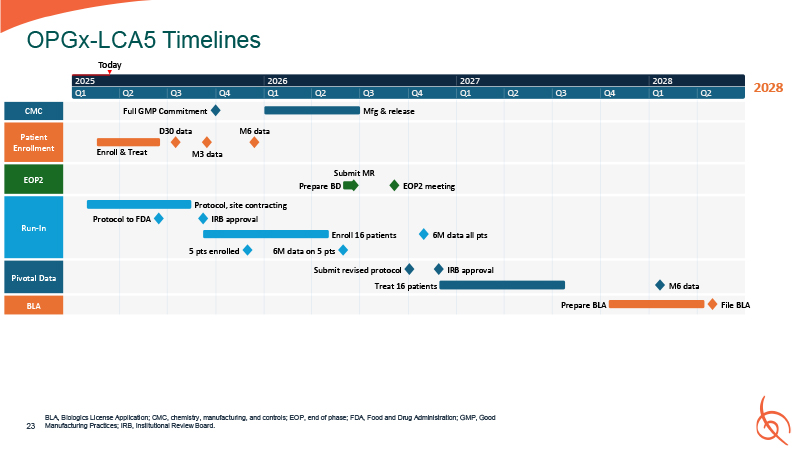
2028 CMC Patient Enrollment EOP2 Run-In Pivotal Data BLA Enroll &
Treat D30 data M3 data M6 data Prepare BD EOP2 meeting Protocol, site contracting Protocol to FDA IRB approval Enroll 16 patients Treat 16 patients Prepare BLA File BLA 5 pts enrolled 6M data on 5 pts Submit revised protocol IRB
approval M6 data 6M data all pts 2025 Q1 Q2 Q3 Full GMP Commitment Q4 2026 Q1 Q2 Q3 Q4 Mfg & release 2027 Q1 Q2 Q3 Q4 2028 Q1 Q2 Submit MR OPGx-LCA5 Timelines Today BLA, Biologics License Application; CMC, chemistry,
manufacturing, and controls; EOP, end of phase; FDA, Food and Drug Administration; GMP, Good Manufacturing Practices; IRB, Institutional Review Board. 23
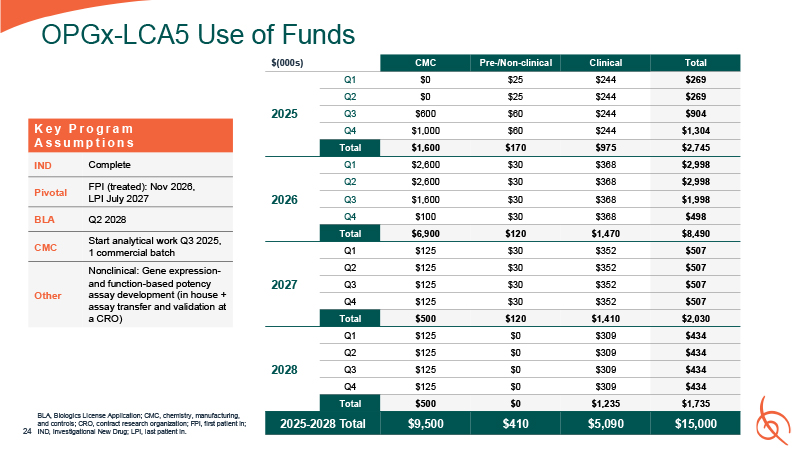
OPGx-LCA5 Use of Funds BLA, Biologics License Application; CMC, chemistry,
manufacturing, and controls; CRO, contract research organization; FPI, first patient in; 24 IND, Investigational New Drug; LPI, last patient
in. $(000s) CMC Pre-/Non-clinical Clinical Total 2025 Q1 $0 $25 $244 $269 Q2 $0 $25 $244 $269 Q3 $600 $60 $244 $904 Q4 $1,000 $60 $244 $1,304 Total $1,600 $170 $975 $2,745 2026 Q1 $2,600 $30 $368 $2,998 Q2 $2,600 $30 $368 $2,998 Q3 $1,600 $30 $368 $1,998 Q4 $100 $30 $368 $498 Total $6,900 $120 $1,470 $8,490 2027 Q1 $125 $30 $352 $507 Q2 $125 $30 $352 $507 Q3 $125 $30 $352 $507 Q4 $125 $30 $352 $507 Total $500 $120 $1,410 $2,030 2028 Q1 $125 $0 $309 $434 Q2 $125 $0 $309 $434 Q3 $125 $0 $309 $434 Q4 $125 $0 $309 $434 Total $500 $0 $1,235 $1,735 2025-2028
Total $9,500 $410 $5,090 $15,000 K e y P r o g r a m A s s u m p t i o n s IND Complete Pivotal FPI (treated): Nov 2026, LPI July 2027 BLA Q2 2028 CMC Start analytical work Q3 2025, 1 commercial batch Other Nonclinical: Gene
expression- and function-based potency assay development (in house + assay transfer and validation at a CRO)

OPGx-BEST1 Phase 1/2-Ready Gene Therapy for BEST1-associated Disease BEST1,
bestrophin 1.
Traditional 5x3 dose escalation is partially skipped if evidence of full-fluid
resolution within 6 months after treatment is seen in Cohort 1 (or Cohort 2) At first occurrence of full fluid resolution in majority (2/3) of a dose cohort, converts to pivotal study extension with 1:1 randomization with crossover (low dose -
1E10 vg/eye) Becomes adaptive Ph 1/2/3 approach like the proposed OPGx-LCA5 adaptive design 26 OPGx-BEST1 Strategy ARB, autosomal recessive BEST1; BEST1, bestrophin 1; BVMD, best vitelliform macular dystrophy. O P G x - B E S T 1 D U O - 1 0
0 1 Design: Phase 1b/2a, open- label, non-randomized, single ascending dose escalation, safety and tolerability study of OPGx-BEST1 in subjects with BVMD or ARB (Basket approach with traditional 3x3 design) Initial dosing: ARB patients
only Up to 3 doses evaluated: 1.4 E9 vg/eye (cohort 1), 4.5 E9 vg/eye (cohort 2), and 2.25 E10 vg/eye (cohort 3); unilaterally injected
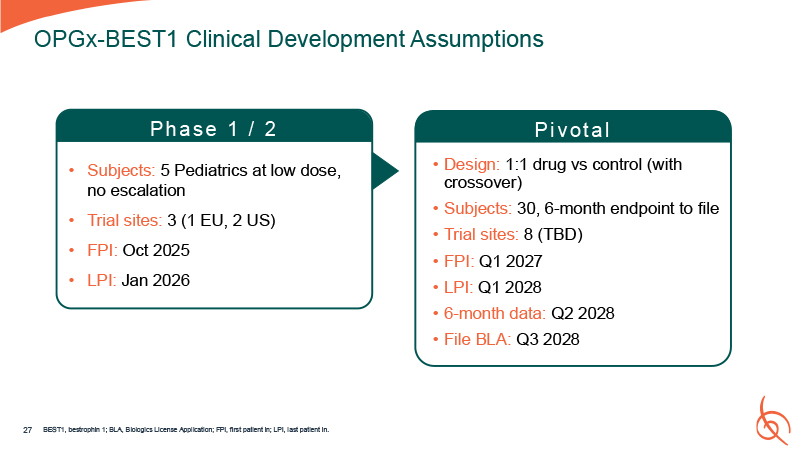
Pi vot a l Design: 1:1 drug vs control (with crossover) Subjects: 30, 6-month
endpoint to file Trial sites: 8 (TBD) FPI: Q1 2027 LPI: Q1 2028 6-month data: Q2 2028 File BLA: Q3 2028 OPGx-BEST1 Clinical Development Assumptions Ph a se 1 / 2 Subjects: 5 Pediatrics at low dose, no escalation Trial sites: 3 (1 EU, 2
US) FPI: Oct 2025 LPI: Jan 2026 27 BEST1, bestrophin 1; BLA, Biologics License Application; FPI, first patient in; LPI, last patient in.
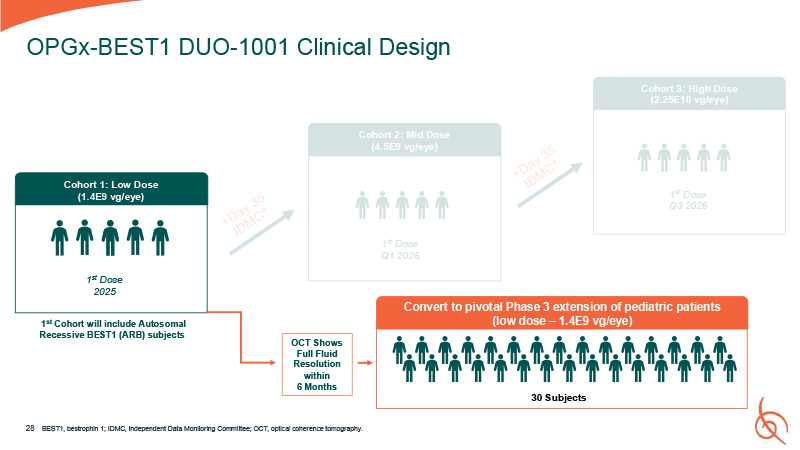
OPGx-BEST1 DUO-1001 Clinical Design Cohort 1: Low Dose (1.4E9 vg/eye) 1st Dose
2025 1st Cohort will include Autosomal Recessive BEST1 (ARB) subjects OCT Shows Full Fluid Resolution within 6 Months Cohort 3: High Dose (2.25E10 vg/eye) Cohort 2: Mid Dose (4.5E9 vg/eye) 1st Dose Q3 2026 1st Dose Q1 2026 28 Convert
to pivotal Phase 3 extension of pediatric patients (low dose – 1.4E9 vg/eye) 30 Subjects BEST1, bestrophin 1; IDMC, Independent Data Monitoring Committee; OCT, optical coherence tomography.
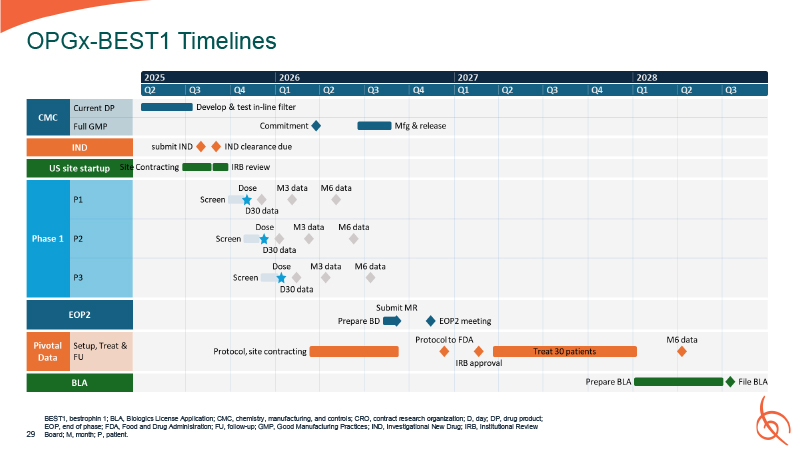
OPGx-BEST1 Timelines BEST1, bestrophin 1; BLA, Biologics License Application; CMC,
chemistry, manufacturing, and controls; CRO, contract research organization; D, day; DP, drug product; EOP, end of phase; FDA, Food and Drug Administration; FU, follow-up; GMP, Good Manufacturing Practices; IND, Investigational New Drug; IRB,
Institutional Review 29 Board; M, month; P, patient.
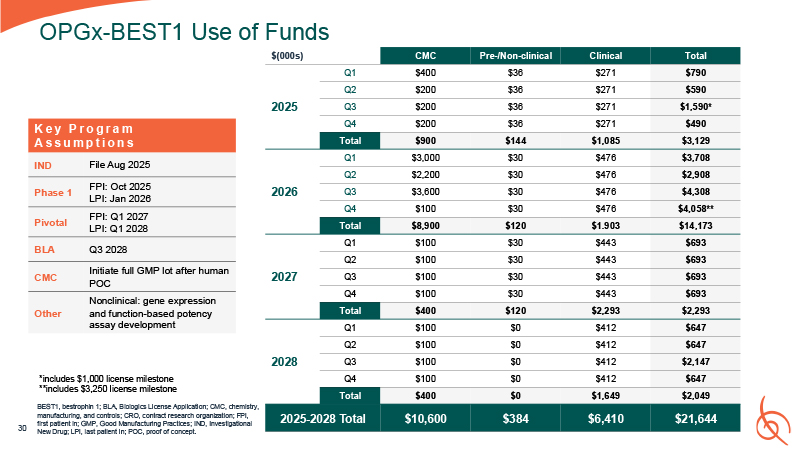
OPGx-BEST1 Use of
Funds 30 $(000s) CMC Pre-/Non-clinical Clinical Total 2025 Q1 $400 $36 $271 $790 Q2 $200 $36 $271 $590 Q3 $200 $36 $271 $1,590* Q4 $200 $36 $271 $490 Total $900 $144 $1,085 $3,129 2026 Q1 $3,000 $30 $476 $3,708 Q2 $2,200 $30 $476 $2,908 Q3 $3,600 $30 $476 $4,308 Q4 $100 $30 $476 $4,058** Total $8,900 $120 $1.903 $14,173 2027 Q1 $100 $30 $443 $693 Q2 $100 $30 $443 $693 Q3 $100 $30 $443 $693 Q4 $100 $30 $443 $693 Total $400 $120 $2,293 $2,293 2028 Q1 $100 $0 $412 $647 Q2 $100 $0 $412 $647 Q3 $100 $0 $412 $2,147 Q4 $100 $0 $412 $647 Total $400 $0 $1,649 $2,049 2025-2028
Total $10,600 $384 $6,410 $21,644 K e y P r o g r a m A s s u m p t i o n s IND File Aug 2025 Phase 1 FPI: Oct 2025 LPI: Jan 2026 Pivotal FPI: Q1 2027 LPI: Q1 2028 BLA Q3 2028 CMC Initiate full GMP lot after human
POC Other Nonclinical: gene expression and function-based potency assay development *includes $1,000 license milestone **includes $3,250 license milestone BEST1, bestrophin 1; BLA, Biologics License Application; CMC, chemistry,
manufacturing, and controls; CRO, contract research organization; FPI, first patient in; GMP, Good Manufacturing Practices; IND, Investigational New Drug; LPI, last patient in; POC, proof of concept.

Timing and Use of Funds OPGx-LCA5 and OPGx-BEST1 BEST1, bestrophin 1; LCA5, Leber
congenital amaurosis 5.
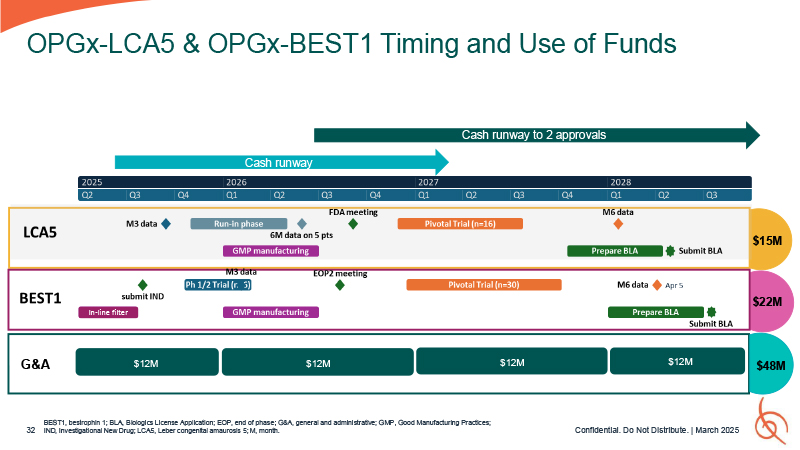
BEST1, bestrophin 1; BLA, Biologics License Application; EOP, end of phase; G&A,
general and administrative; GMP, Good Manufacturing Practices; 32 IND, Investigational New Drug; LCA5, Leber congenital amaurosis 5; M, month. OPGx-LCA5 & OPGx-BEST1 Timing and Use of Funds Cash runway Cash runway to 2
approvals $15M $22M $48M Q1 G&A $12M $12M Confidential. Do Not Distribute. | March 2025 $12M $12M In-line filter

Every patient’ s eyes tell a story Images of real patients with
IRDs. Confidential. Do Not Distribute. | March 2025
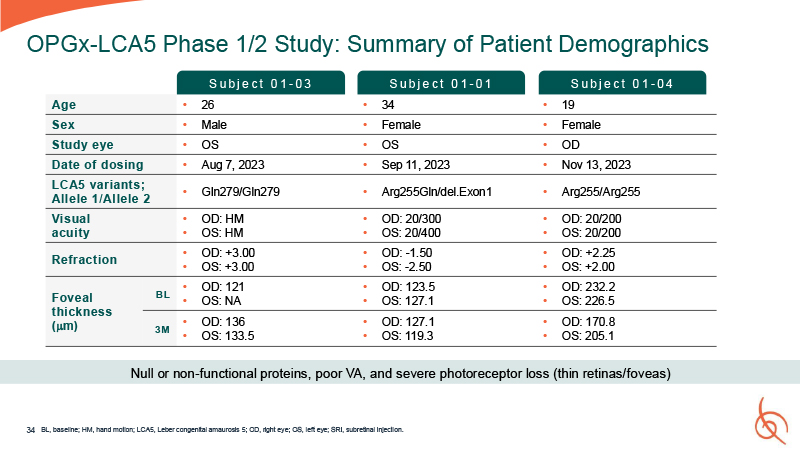
34 OPGx-LCA5 Phase 1/2 Study: Summary of Patient Demographics BL, baseline; HM,
hand motion; LCA5, Leber congenital amaurosis 5; OD, right eye; OS, left eye; SRI, subretinal injection. Age 26 34 19 Sex Male Female Female Study eye OS OS OD Date of dosing Aug 7, 2023 Sep 11, 2023 Nov 13, 2023 LCA5 variants;
Allele 1/Allele 2 Gln279/Gln279 Arg255Gln/del.Exon1 Arg255/Arg255 Visual acuity OD: HM OS: HM OD: 20/300 OS: 20/400 OD: 20/200 OS: 20/200 Refraction OD: +3.00 OS: +3.00 OD: -1.50 OS: -2.50 OD: +2.25 OS: +2.00 Foveal thickness
(m) BL OD: 121 OS: NA OD: 123.5 OS: 127.1 OD: 232.2 OS: 226.5 3M OD: 136 OS: 133.5 OD: 127.1 OS: 119.3 OD: 170.8 OS: 205.1 S u b j e c t 0 1 - 03 S u b j e c t 0 1 - 01 S u b j e c t 0 1 - 04 Null or non-functional proteins,
poor VA, and severe photoreceptor loss (thin retinas/foveas)
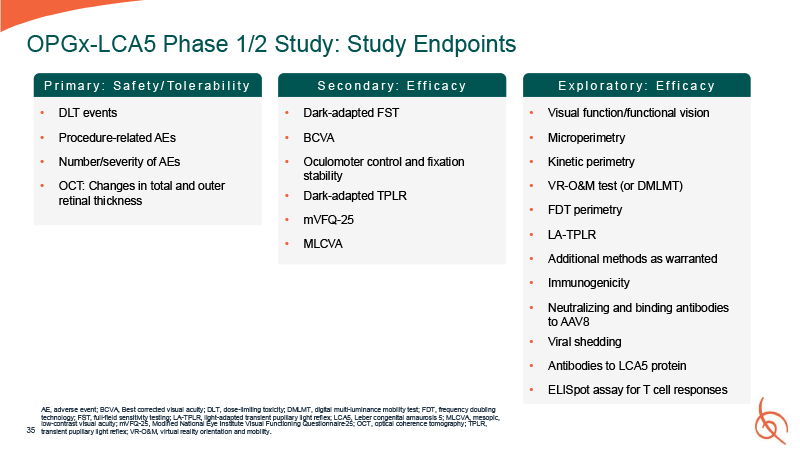
35 OPGx-LCA5 Phase 1/2 Study: Study Endpoints AE, adverse event; BCVA, Best
corrected visual acuity; DLT, dose-limiting toxicity; DMLMT, digital multi-luminance mobility test; FDT, frequency doubling technology; FST, full-field sensitivity testing; LA-TPLR, light-adapted transient pupillary light reflex; LCA5, Leber
congenital amaurosis 5; MLCVA, mesopic, low-contrast visual acuity; mVFQ-25, Modified National Eye Institute Visual Functioning Questionnaire-25; OCT, optical coherence tomography; TPLR, transient pupillary light reflex; VR-O&M, virtual
reality orientation and mobility. Dark-adapted FST BCVA Oculomoter control and fixation stability Dark-adapted TPLR mVFQ-25 MLCVA P r i m a r y : S a f e t y / To l e r a b i l i t y S e c o n d a r y : E f f i c a c y E x p l o r a t o r
y : E f f i c a c y Visual function/functional vision Microperimetry Kinetic perimetry VR-O&M test (or DMLMT) FDT perimetry LA-TPLR Additional methods as warranted Immunogenicity Neutralizing and binding antibodies to AAV8 Viral
shedding Antibodies to LCA5 protein ELISpot assay for T cell responses DLT events Procedure-related AEs Number/severity of AEs OCT: Changes in total and outer retinal thickness
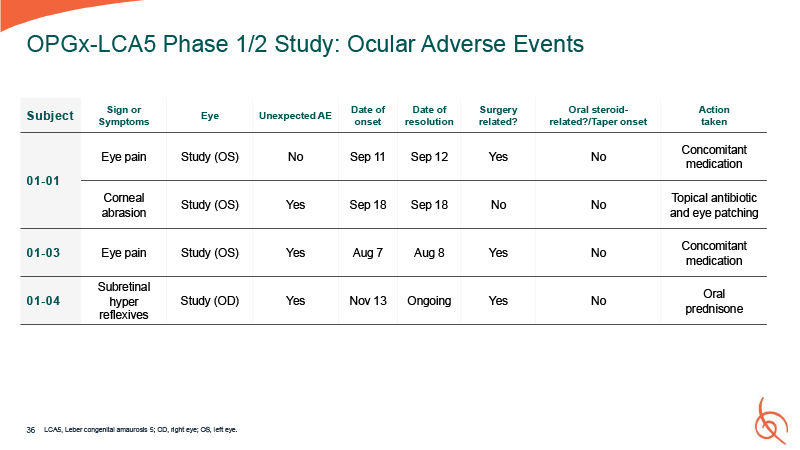
36 OPGx-LCA5 Phase 1/2 Study: Ocular Adverse Events Subject Sign or
Symptoms Eye Unexpected AE Date of onset Date of resolution Surgery related? Oral steroid- related?/Taper onset Action taken 01-01 Eye pain Study (OS) No Sep 11 Sep 12 Yes No Concomitant medication Corneal abrasion Study
(OS) Yes Sep 18 Sep 18 No No Topical antibiotic and eye patching 01-03 Eye pain Study (OS) Yes Aug 7 Aug 8 Yes No Concomitant medication Subretinal 01-04 hyper reflexives Study (OD) Yes Nov 13 Ongoing Yes No Oral
prednisone LCA5, Leber congenital amaurosis 5; OD, right eye; OS, left eye.