EXHIBIT 99.1
Published on September 10, 2015
Exhibit 99.1

Investor Presentation September 2015 1
Safe Harbor Statement The statements that follow (including projections and business trends) are forward-looking statements. Rexahn’s actual results may differ materially from anticipated results and expectations expressed in these forward-looking statements, including as a result of certain risks and uncertainties, such as Rexahn’s lack of profitability, the need for additional capital to operate its business to develop its product candidates; the risk that Rexahn’s development efforts relating to its product candidates may not be successful; the possibility of being unable to obtain regulatory approval of Rexahn’s product candidates; the risk that the results of clinical trials may not be completed on time or support Rexahn’s claims; demand for and market acceptance of Rexahn’s drug candidates; Rexahn’s reliance on third party researchers and manufacturers to develop its product candidates; Rexahn’s ability to develop and obtain protection of its intellectual property; and other risk factors set forth from time to time in our filings with the Securities and Exchange Commission. Rexahn assumes no obligation to update these forward-looking statements. 2
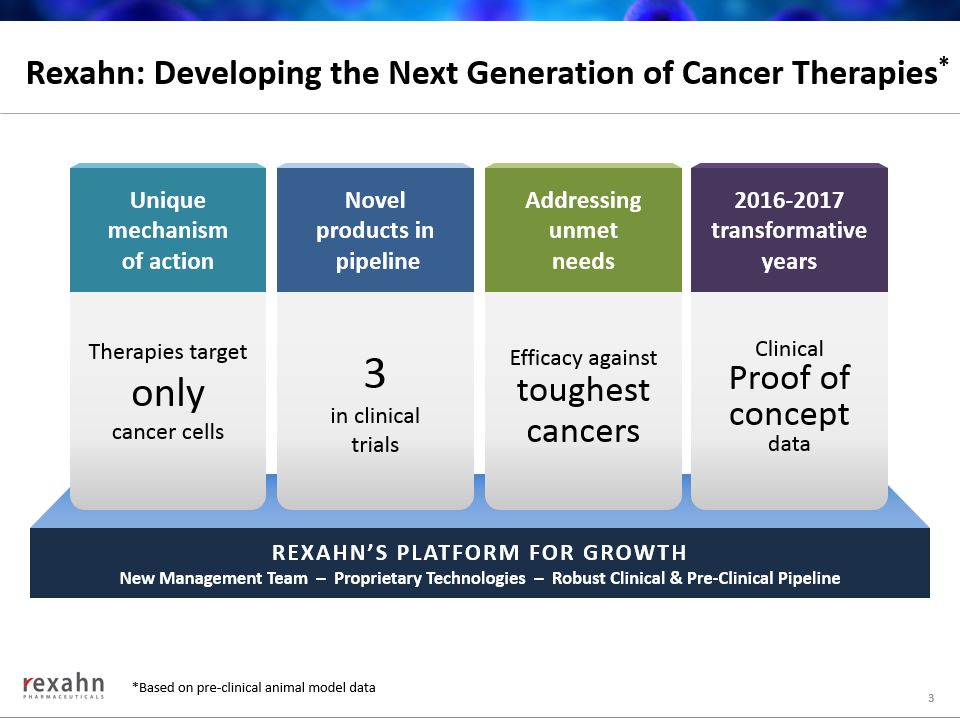
Rexahn: Developing the Next Generation of Cancer Therapies* 3 REXAHN’S PLATFORM FOR GROWTHNew Management Team – Proprietary Technologies – Robust Clinical & Pre-Clinical Pipeline Therapies target onlycancer cells Unique mechanismof action 3in clinicaltrials Novelproducts in pipeline Efficacy against toughest cancers Addressingunmetneeds Clinical Proof of conceptdata 2016-2017transformative years *Based on pre-clinical animal model data
AGENDANext Generation of Cancer Therapies The Company The Pipeline The Future 4

Rexahn: At a Glance Clinical stage biopharmaceutical company developing novel targeted cancer therapeuticsselectively destroy cancer cellsspare normal, healthy cellsHeadquartered in Rockville, MarylandNYSE MKT: RNNMarket cap: $105M7% owned by management/insidersCash and investments at June 30, 2015: $26.0MEstimated quarterly burn rate: ~4.0MGAAP net loss for the three months ended June 30, 2015: $(0.02) 5

New, Experienced Leadership Team – Built in Last 2 Years Reza Mazhari, Vice President, Translational Medicine Experienced pharmaceutical executive; success taking multiple compounds from concept to clinic Co-Founder of Cardioxyl Pharmaceuticals, VP, Drug Discovery and Development at Cerecor Ted Jeong D.Mgt., Sr. Vice President and Chief Financial Officer Extensive experience in venture capital and investment bankingOversees all aspects of capital raising, accounting, operations, and corporate development Ely Benaim M.D., Chief Medical Officer 25+ years experience in healthcare including 15 years of clinical research experience in academia, government and pharmaceutical industryExtensive experience in global regulatory affairs Peter Suzdak Ph.D., Chief Executive Officer 25+ years experience in the biopharmaceutical industryBroad experience spanning pre-clinical, development and commercialization; 18 IND filings, 3 NDA submissions
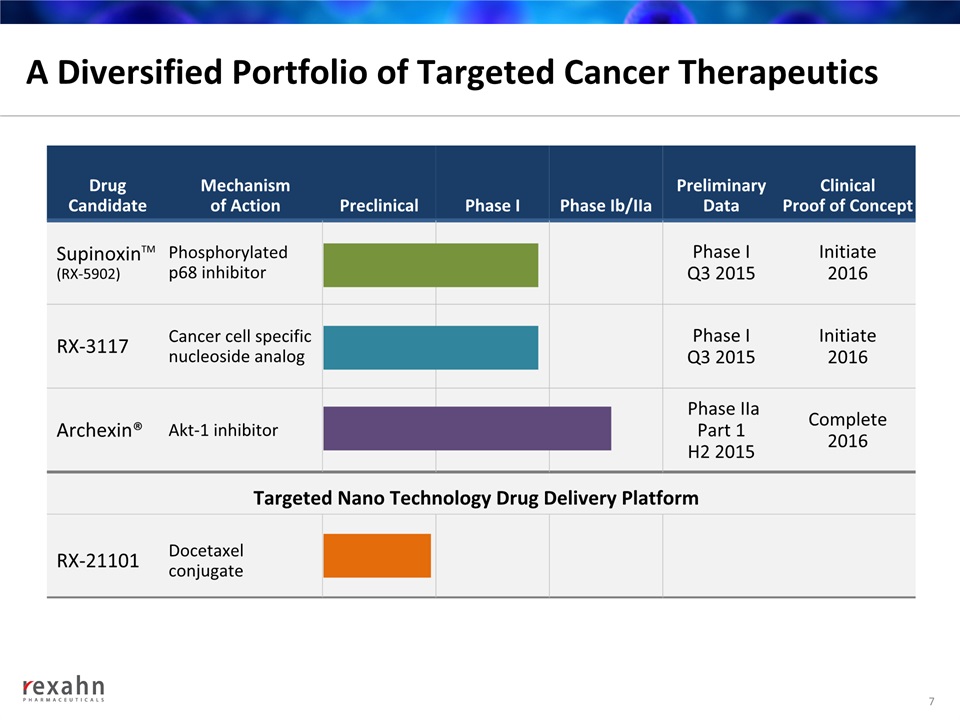
A Diversified Portfolio of Targeted Cancer Therapeutics DrugCandidate Mechanismof Action Preclinical Phase I Phase Ib/IIa Preliminary Data Clinical Proof of Concept SupinoxinTM (RX-5902) Phosphorylated p68 inhibitor Phase IQ3 2015 Initiate 2016 RX-3117 Cancer cell specific nucleoside analog Phase I Q3 2015 Initiate 2016 Archexin® Akt-1 inhibitor Phase IIa Part 1 H2 2015 Complete 2016 RX-21101 Docetaxelconjugate Targeted Nano Technology Drug Delivery Platform 7
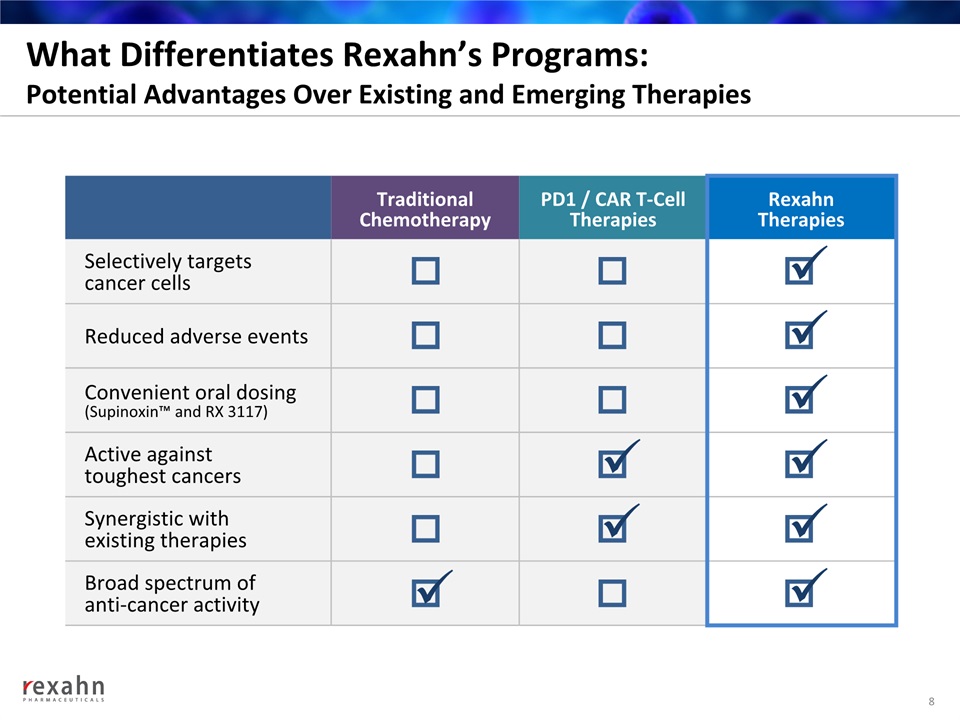
What Differentiates Rexahn’s Programs: Potential Advantages Over Existing and Emerging Therapies 8 Traditional Chemotherapy PD1 / CAR T-CellTherapies RexahnTherapies Selectively targets cancer cells Reduced adverse events Convenient oral dosing(Supinoxin™ and RX 3117) Active against toughest cancers Synergistic with existing therapies Broad spectrum of anti-cancer activity ü ü ü ü ü ü ü ü ü
AGENDANext Generation of Cancer Therapies The Company The Pipeline The Future 9
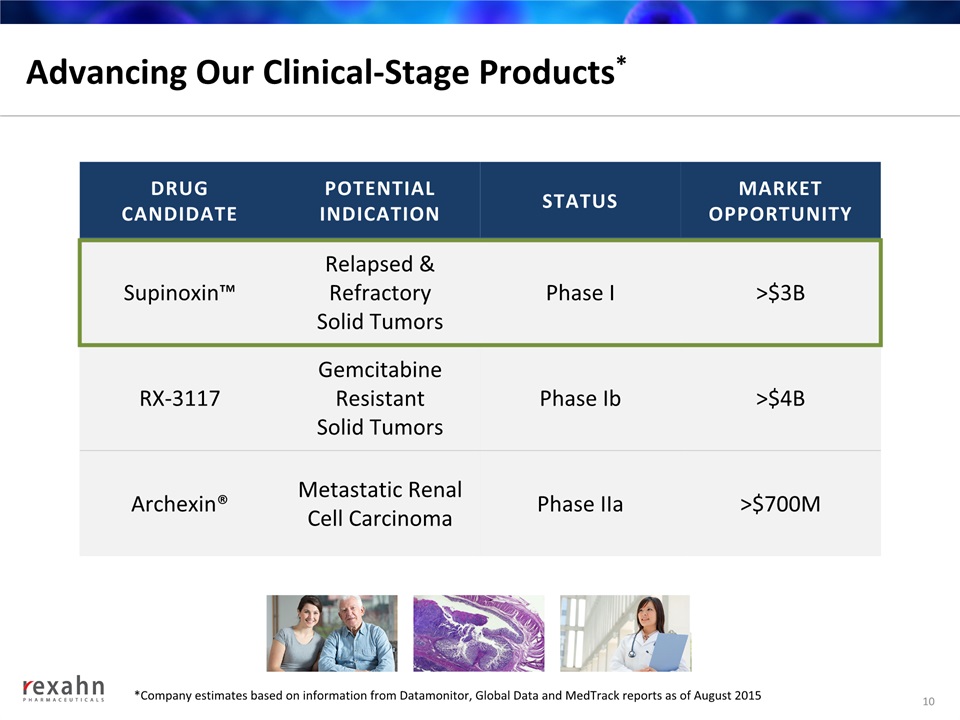
Advancing Our Clinical-Stage Products* 10 DRUGCANDIDATE POTENTIALINDICATION STATUS MARKETOPPORTUNITY Supinoxin™ Relapsed & Refractory Solid Tumors Phase I >$3B RX-3117 Gemcitabine Resistant Solid Tumors Phase Ib >$4B Archexin® Metastatic Renal Cell Carcinoma Phase IIa >$700M *Company estimates based on information from Datamonitor, Global Data and MedTrack reports as of August 2015
SUPINOXIN™ OVERVIEWPotential First-in-Class Inhibitor of a Unique Cancer Protein The CandidateOrally active, highly potent small molecule inhibitor of phosphorylated p68 (p-p68)Significant Unmet Medical NeedDemonstrated activity in >100 human cancer cell lines including: triple-negative breast, colon, ovarian, pancreas, non small cell lung cancer, and renalClinical Development – Status Phase I clinical trial with Supinoxin™ in cancer patients is ongoingPreliminary data expected Q3 2015Initiate Clinical Proof-of-Concept study in 2016Commercial PotentialPotential market opportunity: >$3BStrong intellectual property protectionOngoing corporate partnership discussions 11
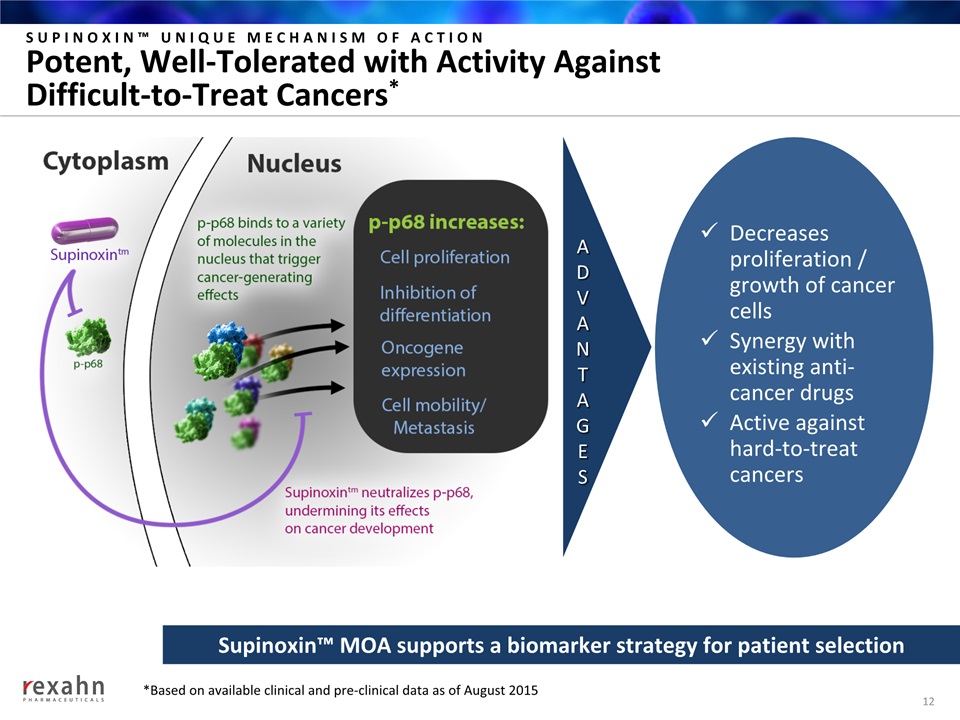
SUPINOXIN™ UNIQUE MECHANISM OF ACTIONPotent, Well-Tolerated with Activity Against Difficult-to-Treat Cancers* 12 Decreases proliferation / growth of cancer cellsSynergy with existing anti-cancer drugsActive against hard-to-treat cancers Supinoxin™ MOA supports a biomarker strategy for patient selection ADVANTAGES *Based on available clinical and pre-clinical data as of August 2015
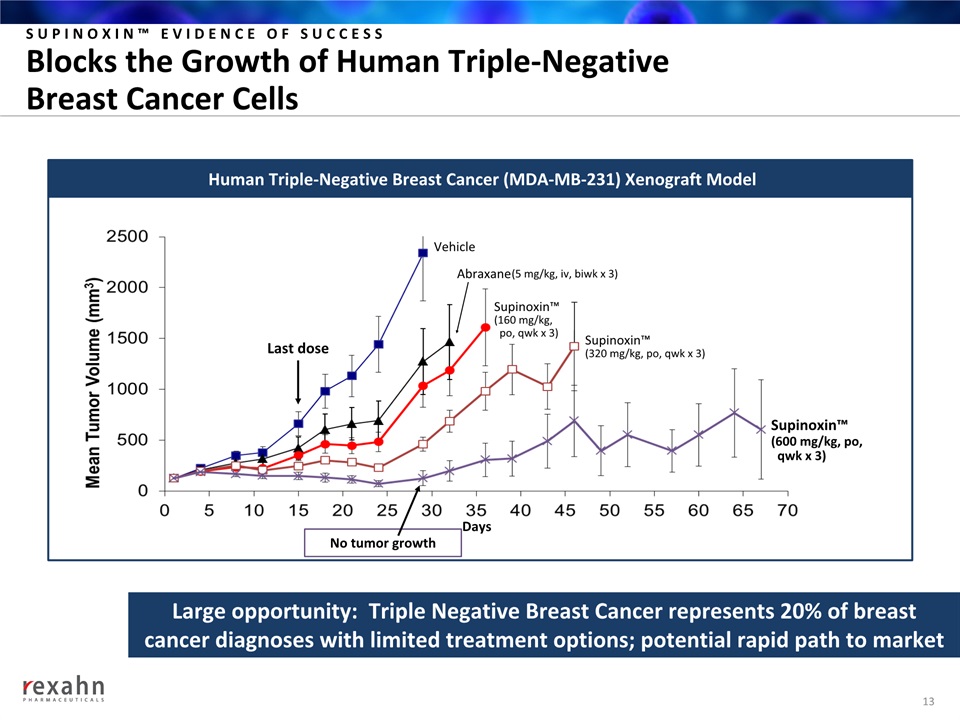
SUPINOXIN™ EVIDENCE OF SUCCESSBlocks the Growth of Human Triple-Negative Breast Cancer Cells 13 Large opportunity: Triple Negative Breast Cancer represents 20% of breast cancer diagnoses with limited treatment options; potential rapid path to market Human Triple-Negative Breast Cancer (MDA-MB-231) Xenograft Model Days Last dose Supinoxin™(160 mg/kg, po, qwk x 3) Supinoxin™(320 mg/kg, po, qwk x 3) Supinoxin™(600 mg/kg, po, qwk x 3) Abraxane (5 mg/kg, iv, biwk x 3) Vehicle No tumor growth
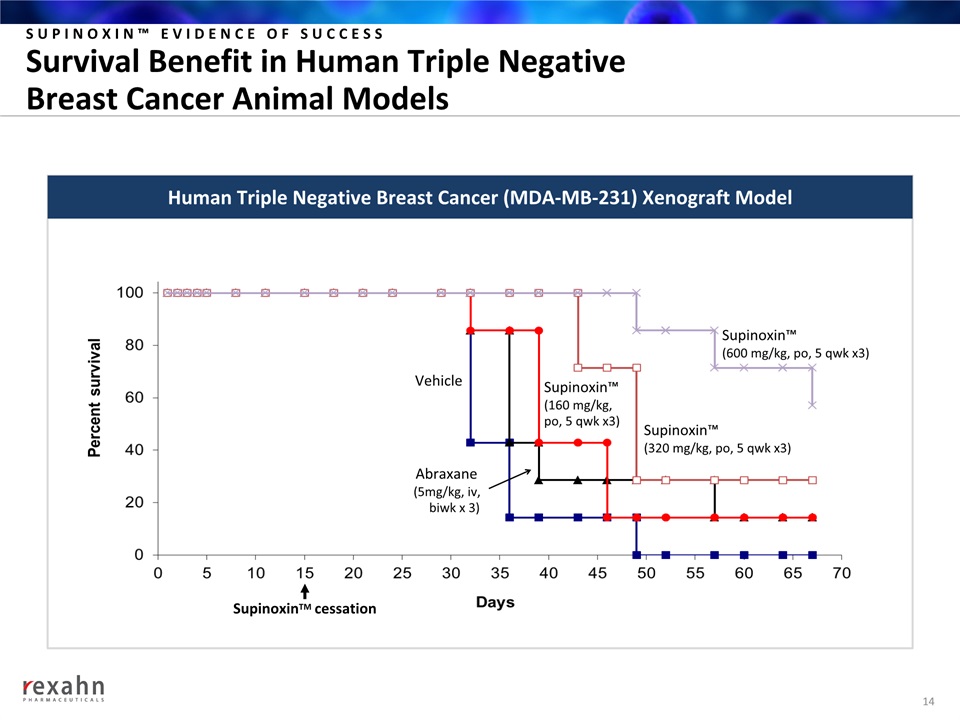
SUPINOXIN™ EVIDENCE OF SUCCESSSurvival Benefit in Human Triple Negative Breast Cancer Animal Models 14 SupinoxinTM cessation Human Triple Negative Breast Cancer (MDA-MB-231) Xenograft Model Supinoxin™ (600 mg/kg, po, 5 qwk x3) Abraxane (5mg/kg, iv, biwk x 3) Vehicle Supinoxin™ (320 mg/kg, po, 5 qwk x3) Supinoxin™ (160 mg/kg, po, 5 qwk x3)
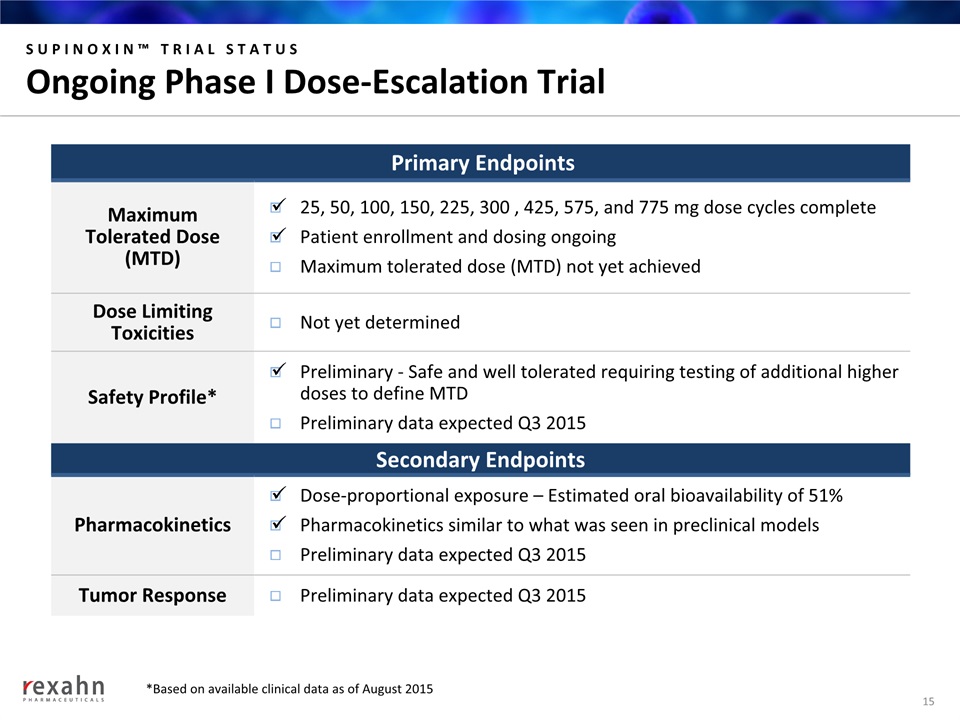
SUPINOXIN™ TRIAL STATUSOngoing Phase I Dose-Escalation Trial 15 Primary Endpoints MaximumTolerated Dose(MTD) 25, 50, 100, 150, 225, 300 , 425, 575, and 775 mg dose cycles completePatient enrollment and dosing ongoingMaximum tolerated dose (MTD) not yet achieved Dose Limiting Toxicities Not yet determined Safety Profile* Preliminary - Safe and well tolerated requiring testing of additional higher doses to define MTDPreliminary data expected Q3 2015 Secondary Endpoints Pharmacokinetics Dose-proportional exposure – Estimated oral bioavailability of 51%Pharmacokinetics similar to what was seen in preclinical modelsPreliminary data expected Q3 2015 Tumor Response Preliminary data expected Q3 2015 *Based on available clinical data as of August 2015
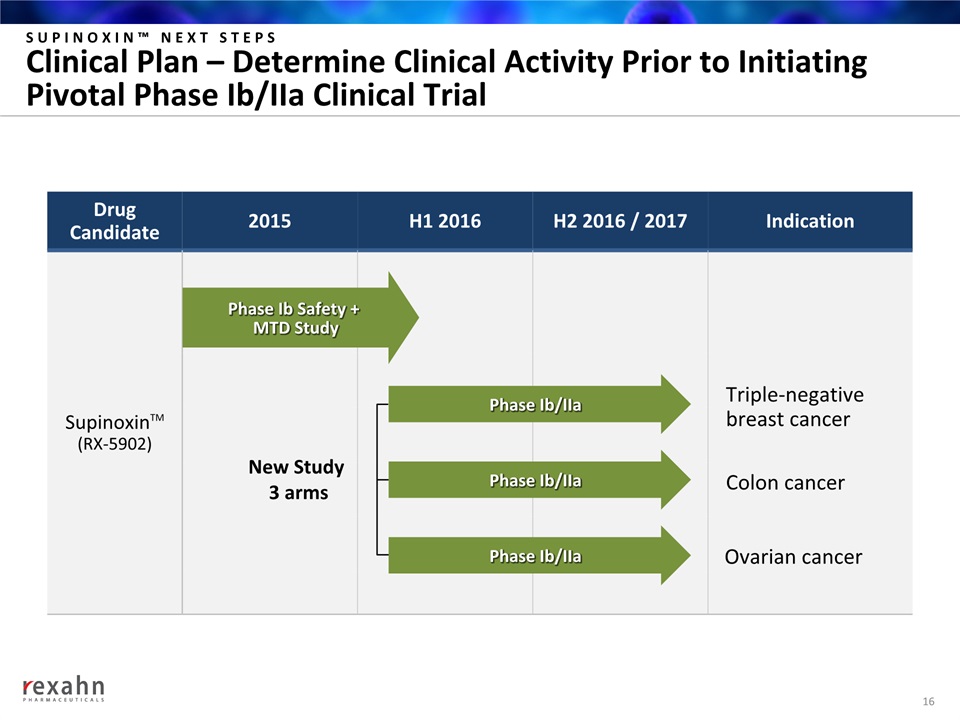
SUPINOXIN™ NEXT STEPSClinical Plan – Determine Clinical Activity Prior to Initiating Pivotal Phase Ib/IIa Clinical Trial 16 DrugCandidate 2015 H1 2016 H2 2016 / 2017 Indication SupinoxinTM(RX-5902) Phase Ib/IIa Phase Ib/IIa Phase Ib/IIa Phase Ib Safety + MTD Study New Study 3 arms Triple-negativebreast cancer Colon cancer Ovarian cancer

Advancing Our Clinical-Stage Products* 17 DRUGCANDIDATE POTENTIALINDICATION STATUS MARKET OPPORTUNITY Supinoxin™ Relapsed & Refractory Solid Tumors Phase I >$3B RX-3117 Gemcitabine Resistant Solid Tumors Phase Ib >$4B Archexin® Metastatic Renal Cell Carcinoma Phase IIa >$700M *Company estimates based on information from Datamonitor, Global Data and MedTrack reports as of August 2015
RX-3117 OVERVIEWNovel Next Generation Nucleoside Compound The CandidateCancer cell specific small molecule nucleoside analogue that inhibits DNA and RNA synthesis causing cell death Prodrug activated by UCK2 which is only present in cancer cellsActive following oral administrationSignificant Unmet Medical NeedGemcitabine-resistant cancers: bladder, colon, pancreatic, non-small cell lung cancer, renal and other solid tumorsClinical Development – StatusCompleted Phase I trial confirming oral bioavailability and initial safetyPhase Ib clinical trial in cancer patients is ongoingPreliminary data expected Q3 2015Initiate Clinical Proof-of-Concept study in 2016Commercial PotentialPotential market opportunity: >$4BStrong intellectual property portfolioOngoing partnership discussions 18
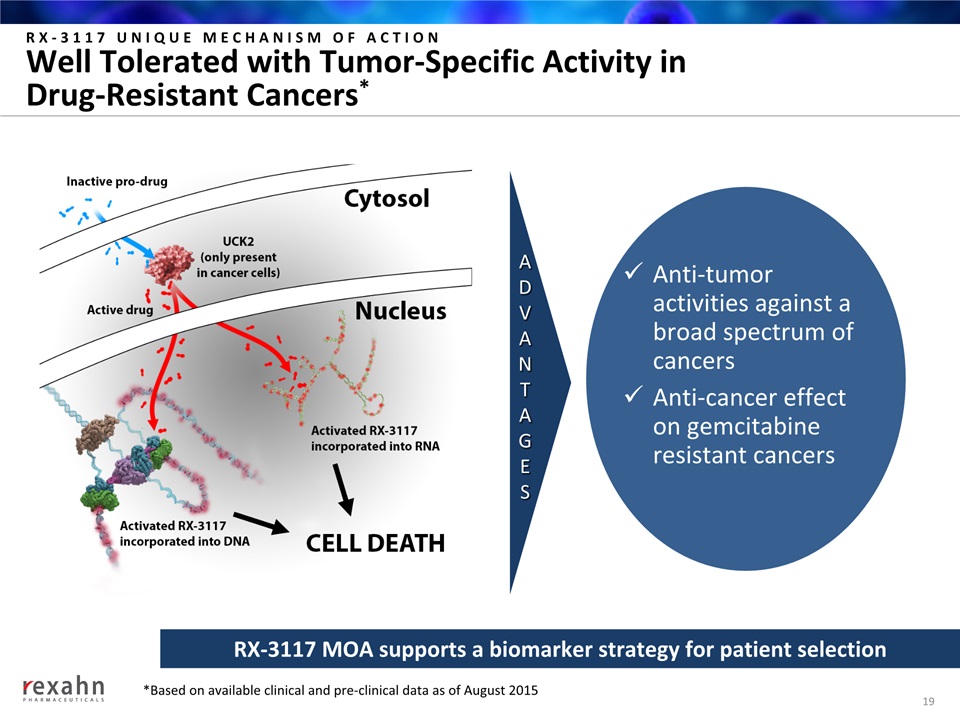
Anti-tumor activities against a broad spectrum of cancersAnti-cancer effect on gemcitabine resistant cancers RX-3117 UNIQUE MECHANISM OF ACTIONWell Tolerated with Tumor-Specific Activity in Drug-Resistant Cancers* 19 RX-3117 MOA supports a biomarker strategy for patient selection ADVANTAGES *Based on available clinical and pre-clinical data as of August 2015
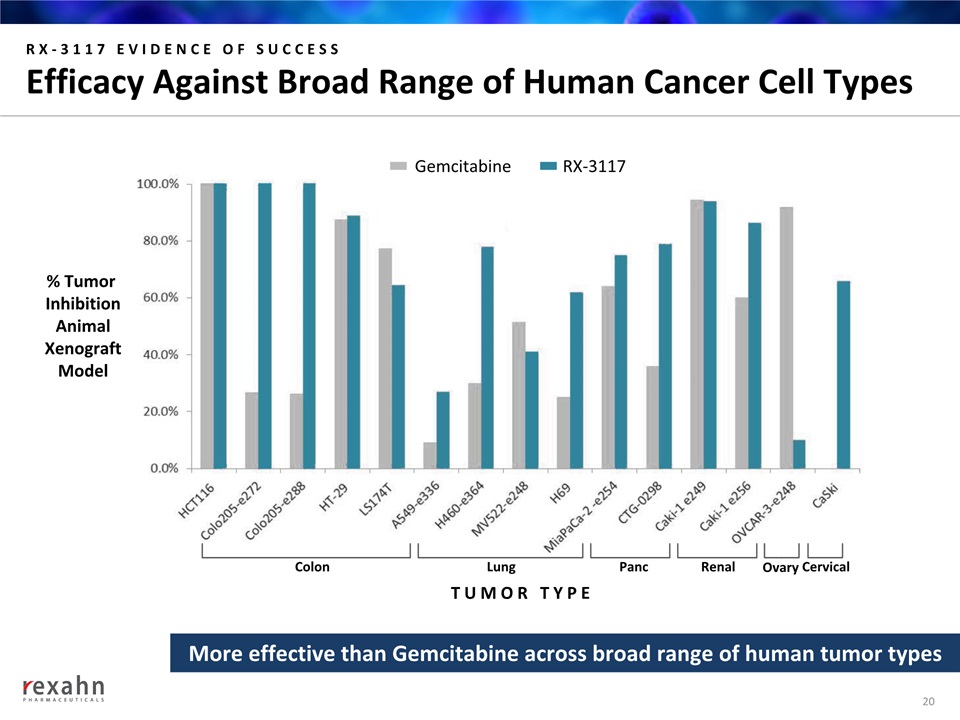
RX-3117 EVIDENCE OF SUCCESSEfficacy Against Broad Range of Human Cancer Cell Types 20 % Tumor InhibitionAnimalXenograftModel Colon Lung Panc Renal Ovary Cervical TUMOR TYPE More effective than Gemcitabine across broad range of human tumor types RX-3117 Gemcitabine
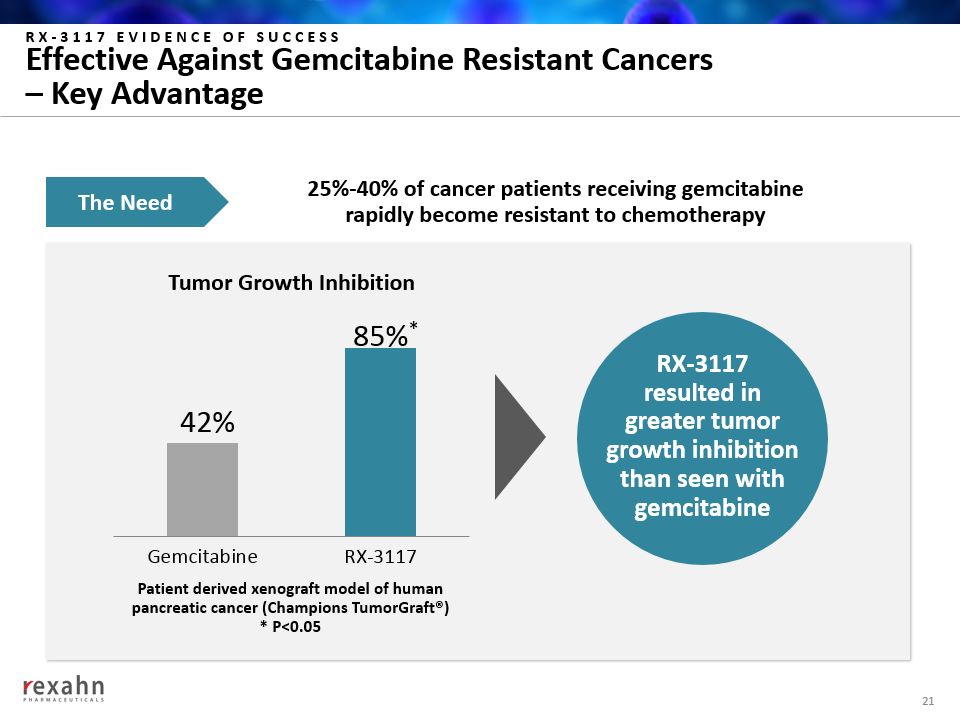
Tumor Growth Inhibition RX-3117 EVIDENCE OF SUCCESSEffective Against Gemcitabine Resistant Cancers – Key Advantage 21 25%-40% of cancer patients receiving gemcitabinerapidly become resistant to chemotherapy RX-3117resulted ingreater tumorgrowth inhibitionthan seen withgemcitabine Patient derived xenograft model of human pancreatic cancer (Champions TumorGraft®)* P<0.05 The Need
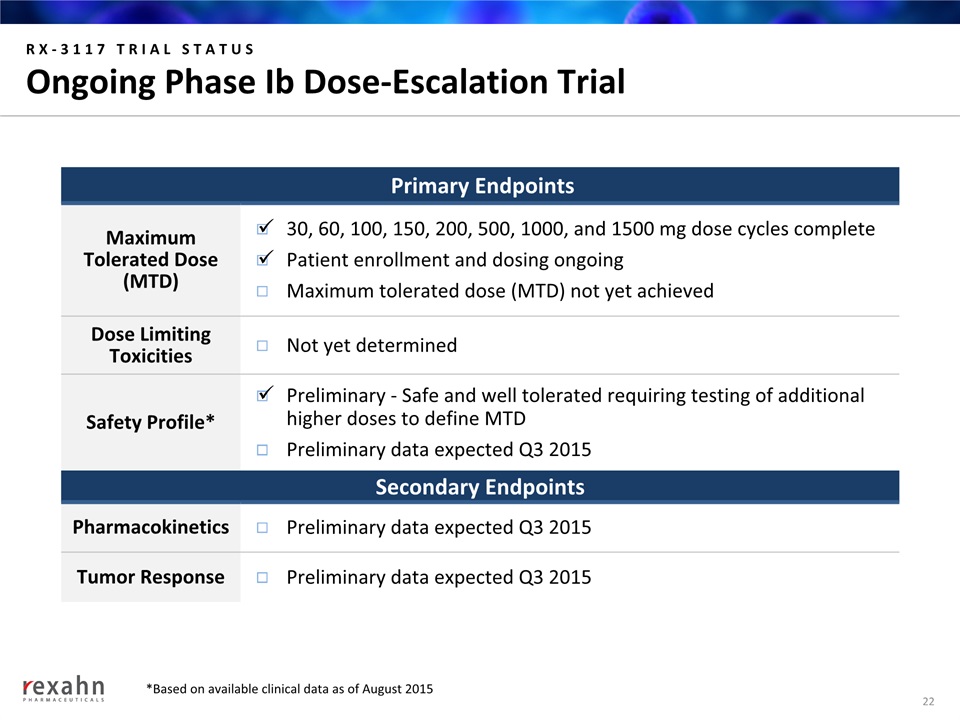
RX-3117 TRIAL STATUSOngoing Phase Ib Dose-Escalation Trial Primary Endpoints MaximumTolerated Dose(MTD) 30, 60, 100, 150, 200, 500, 1000, and 1500 mg dose cycles completePatient enrollment and dosing ongoingMaximum tolerated dose (MTD) not yet achieved Dose Limiting Toxicities Not yet determined Safety Profile* Preliminary - Safe and well tolerated requiring testing of additional higher doses to define MTDPreliminary data expected Q3 2015 Secondary Endpoints Pharmacokinetics Preliminary data expected Q3 2015 Tumor Response Preliminary data expected Q3 2015 22 *Based on available clinical data as of August 2015
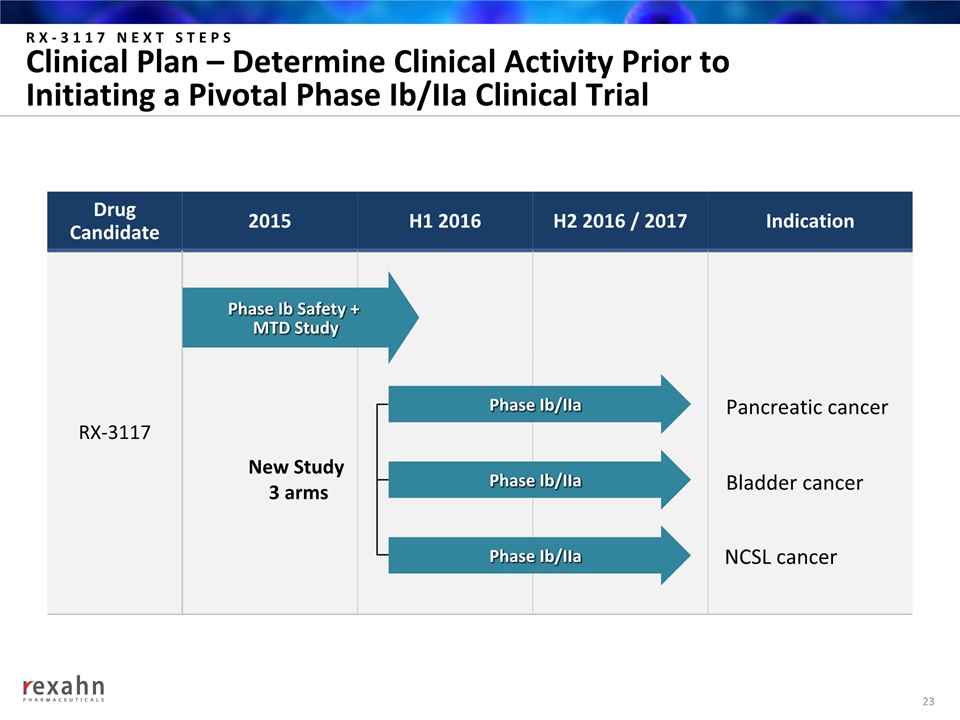
RX-3117 NEXT STEPSClinical Plan – Determine Clinical Activity Prior to Initiating a Pivotal Phase Ib/IIa Clinical Trial 23 DrugCandidate 2015 H1 2016 H2 2016 / 2017 Indication RX-3117 Phase Ib/IIa Phase Ib/IIa Phase Ib/IIa Phase Ib Safety + MTD Study New Study 3 arms Pancreatic cancer Bladder cancer NCSL cancer

Advancing Our Clinical-Stage Products* DRUGCANDIDATE POTENTIAL INDICATION STATUS MARKET OPPORTUNITY Supinoxin™ Relapsed & Refractory Solid Tumors Phase I >$3B RX-3117 Gemcitabine resistant Solid Tumors Phase Ib >$4B Archexin® Metastatic Renal Cell Carcinoma Phase IIa >$700M 24 *Company estimates based on information from Datamonitor, Global Data and MedTrack reports as of August 2015

ARCHEXIN® OVERVIEWPotential Best-in-Class AKT-1 Inhibitor The CandidateNovel inhibitor of cancer cell signaling protein, Akt-1, increasing cancer cell deathTargets clinically validated cancer pathwayAlso inhibits drug resistance; synergistic with approved drugsSignificant Unmet Medical NeedCurrently targeting metastatic renal cell carcinoma (mRCC) Clinical Development – StatusCompleted Phase I trial in cancer patientsPancreatic cancer- Phase IIa completedPhase IIa trial in metastatic RCC – ongoingInitial combination safety data mid 2015Commercial PotentialFDA orphan drug designation for 5 cancers (renal, glioblastoma, ovarian, stomach, pancreas)Potential market opportunity: >$700MStrong intellectual property portfolioOngoing partnership discussions 25
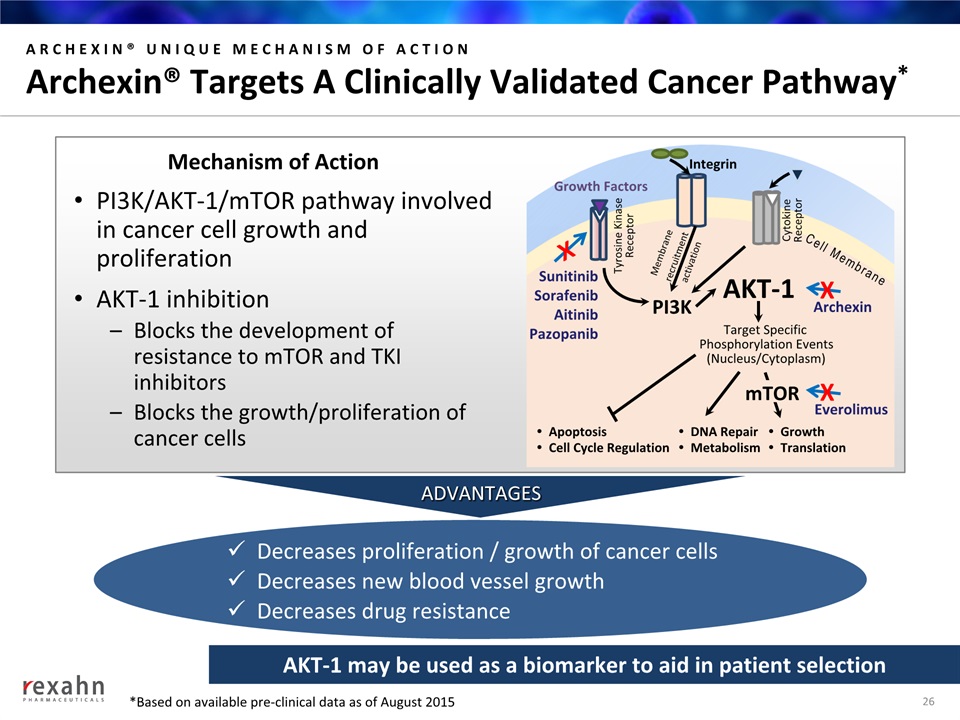
ARCHEXIN® UNIQUE MECHANISM OF ACTIONArchexin® Targets A Clinically Validated Cancer Pathway* PI3K/AKT-1/mTOR pathway involved in cancer cell growth and proliferationAKT-1 inhibitionBlocks the development of resistance to mTOR and TKI inhibitorsBlocks the growth/proliferation of cancer cells Decreases proliferation / growth of cancer cellsDecreases new blood vessel growthDecreases drug resistance Mechanism of Action ADVANTAGES 26 PI3K Growth Factors AKT-1 Target SpecificPhosphorylation Events(Nucleus/Cytoplasm) ApoptosisCell Cycle Regulation DNA RepairMetabolism GrowthTranslation mTOR SunitinibSorafenibAitinibPazopanib Everolimus Archexin X Cell Membrane Tyrosine KinaseReceptor Integrin Membrane recruitmentactivation CytokineReceptor X X AKT-1 may be used as a biomarker to aid in patient selection *Based on available pre-clinical data as of August 2015
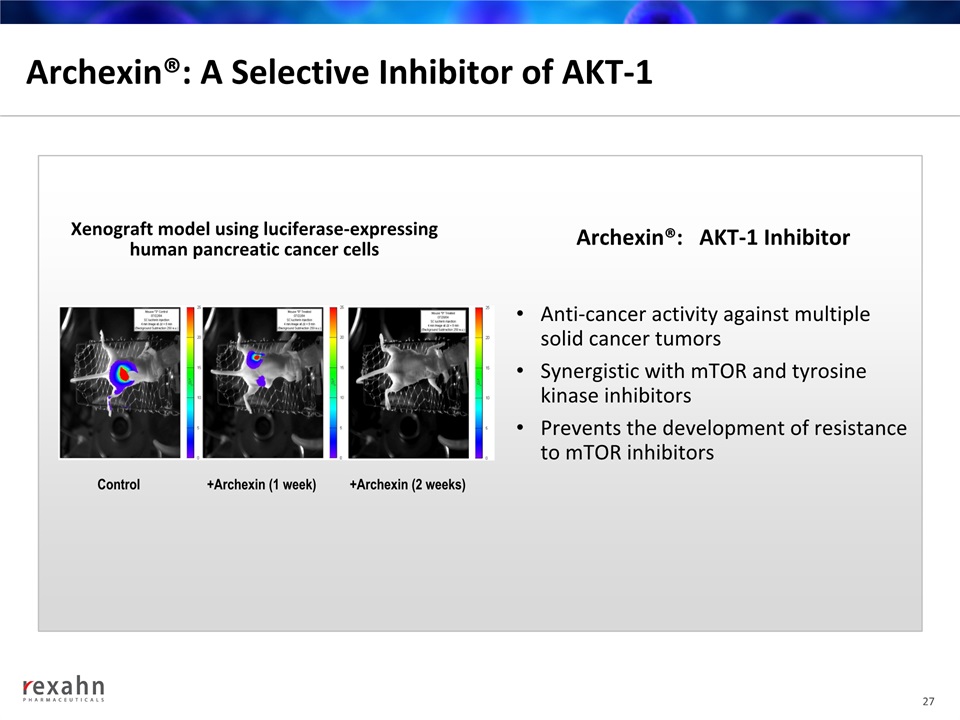
Archexin®: A Selective Inhibitor of AKT-1 27 Xenograft model using luciferase-expressinghuman pancreatic cancer cells Control +Archexin (1 week) +Archexin (2 weeks) Anti-cancer activity against multiple solid cancer tumorsSynergistic with mTOR and tyrosine kinase inhibitorsPrevents the development of resistance to mTOR inhibitors Archexin®: AKT-1 Inhibitor
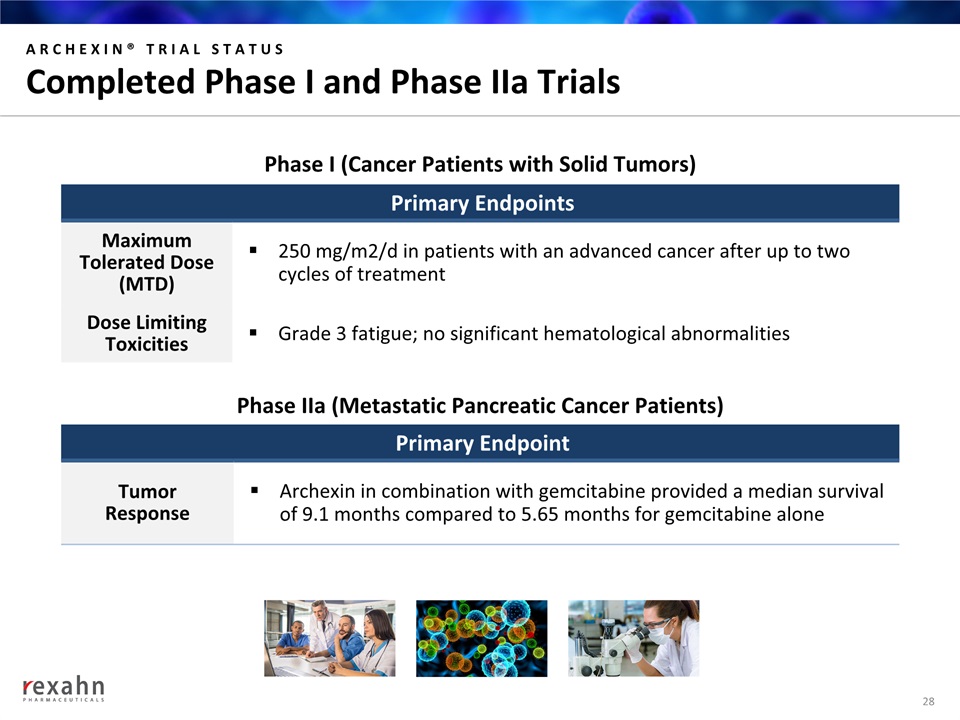
ARCHEXIN® TRIAL STATUSCompleted Phase I and Phase IIa Trials 28 Primary Endpoints MaximumTolerated Dose(MTD) 250 mg/m2/d in patients with an advanced cancer after up to two cycles of treatment Dose Limiting Toxicities Grade 3 fatigue; no significant hematological abnormalities Phase I (Cancer Patients with Solid Tumors) Primary Endpoint TumorResponse Archexin in combination with gemcitabine provided a median survival of 9.1 months compared to 5.65 months for gemcitabine alone Phase IIa (Metastatic Pancreatic Cancer Patients)

29 Phase IIa StudyDesign Metastatic renal cell carcinoma (mRCC)Second line therapyAdministered in combination with everolimus (Affinitor®)Part A: Identify maximum tolerated dose in combination with everolimus Part B: Determine safety and efficacy in 30 additional mRCC patients ARCHEXIN® STATUS AND NEXT STEPSOnly Selective AKT-1 Inhibitor in Clinical Development - Status
AGENDANext Generation of Cancer Therapies The Company The Pipeline The Future 30
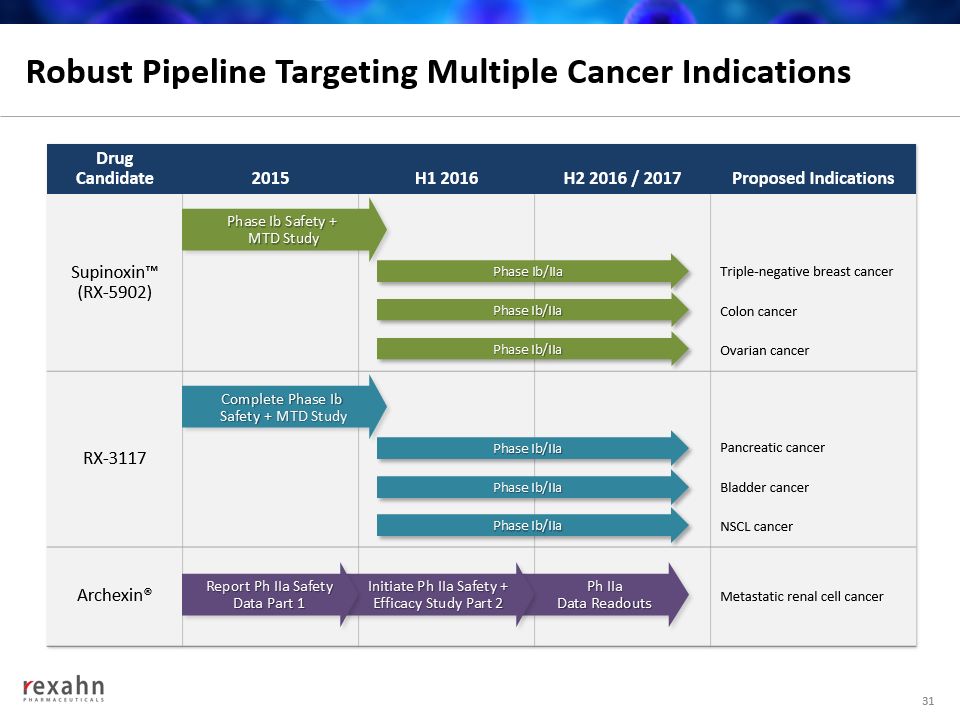
Robust Pipeline Targeting Multiple Cancer Indications 31 DrugCandidate 2015 H1 2016 H2 2016 / 2017 Proposed Indications Supinoxin™(RX-5902) Triple-negative breast cancer Colon cancer Ovarian cancer RX-3117 Pancreatic cancer Bladder cancer NSCL cancer Archexin® Metastatic renal cell cancer Phase Ib/IIa Phase Ib/IIa Phase Ib/IIa Phase Ib/IIa Phase Ib/IIa Phase Ib/IIa Phase Ib Safety + MTD Study Complete Phase Ib Safety + MTD Study Ph IIaData Readouts Initiate Ph IIa Safety +Efficacy Study Part 2 Report Ph IIa SafetyData Part 1
Advance cancer therapies through proof-of-concept clinical development Establish partnerships with pharmaceutical companies; focus on maximizing shareholder value Advance pre-clinical oncology programs to address significant unmet needs 32

Investor Presentation September 2015 33