EXHIBIT 99.2
Published on November 6, 2020
Exhibit 99.2

Ocuphire Corporate Presentation November 2020

Disclosures and Forward Looking Statements 2 This presentation contains “forward-looking
statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Such statements include, but are not limited to, statements concerning Ocuphire Pharma, Inc.’s (“Ocuphire” or the “Company”) product candidates and
potential. These forward-looking statements are based upon the Company’s current expectations and involve assumptions that may never materialize or may prove to be incorrect. Actual results and the timing of events could differ materially
from those anticipated in such forward-looking statements as a result of various risks and uncertainties, including, without limitation:(i) potential adverse reactions or changes to business relationships resulting from the announcement
or completion of the merger; (ii) the success and timing of regulatory submissions and pre-clinical and clinical trials; (iii) regulatory requirements or developments; (iv) changes to clinical trial designs and regulatory pathways;(v)
changes in capital resource requirements; (vi) risks related to the inability of the Company to obtain sufficient additional capital to continue to advance its product candidates and its preclinical programs; (vii) legislative,
regulatory, political and economic developments, and (viii) the effects of COVID-19 on clinical programs and business operations. The foregoing review of important factors that could cause actual events to differ from expectations should
not be construed as exhaustive and should be read in conjunction with statements that are included herein and elsewhere, including the risk factors detailed in documents that have been and may be filed by the Company from time to time
with the SEC (including the proxy statement/prospectus included in that certain Registration Statement on Form S-4 (File No. 333-239702) initially filed with the SEC on July 6, 2020 and declared effective by the SEC on October 2, 2020).
All forward-looking statements contained in this presentation speak only as of the date on which they were made. The Company undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after
the date on which they were made.The Company makes no representation or warranty, express or implied, as to the accuracy or completeness of the information contained in or incorporated by reference into this presentation. Nothing
contained in or incorporated by reference into this presentation is, or shall be relied upon as, a promise or representation by the Company as to the past or future. The Company assumes no responsibility for the accuracy or completeness
of any such information. This presentation may not be reproduced or provided to any other person (other than your advisor) without our prior written consent. By accepting delivery of this presentation, you agree to the foregoing and agree
to return this presentation and any documents related thereto and any copies thereof to us or to destroy the same if you do not make an investment in any securities. The information contain within this presentation shall not, except as
hereinafter provided, without the prior written consent of the Company, be disclosed by you or your representatives in any manner whatsoever, in whole or in part, and shall not be used by you or your representatives other than for the
purpose of evaluating the transaction described herein. By accepting delivery of this presentation you further acknowledge and agree aware of the restrictions imposed by the United States securities laws on the purchase or sale of
securities by any person who has received material, nonpublic information from the issuer of the securities or any affiliate thereof and on the communication of such information to any other person when it is reasonably foreseeable that
such other person is likely to purchase or sell such securities in reliance on such information for so long as the information remains material and non-public. This presentation also contains estimates and other statistical data made by
independent parties and by us relating to market shares and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. The trademarks
included herein are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of such products.

Ocuphire - New Public Ophthalmic Drug Development CompanyNasdaq Symbol: OCUP Nasdaq public
listing allows for capital access, investor liquidity, and strategic visibilityReverse merger with Rexahn and financing transaction announced in June, closed in NovemberOcuphire management team, expanded board and field-leading SABNo
Rexahn legacy operating/capital obligations (CVR for Rexahn shareholders on existing agreements with Biosense and Haichang)REXN represented by Oppenheimer for the mergerConcurrent $21+ million PIPE financing led by Altium CapitalPro forma
cash provides sufficient capital to fund 4 late-stage clinical trials through their respective readouts in 2021Financing co-led by Cantor Fitzgerald and Canaccord GenuityOcuphire focusing exclusively on ophthalmic drug developmentTwo lead
assets: Nyxol Eye Drops and APX3330 oral tabletsTwo Phase 3 trials and two Phase 2 trials expected to readout in 2021 November 6, 2020 3

Ocuphire Management TeamDecades of Biotech and Drug Development Experience Mina
Sooch, MBA Chief Executive Officer 25 years in pharma/biotech industry as a CEO, entrepreneur, venture capitalist and strategist. Raised over 100 million dollars for 2 biotechs, then led private to public on Nasdaq, Recognized as MI
Newsmaker of the Year. Konstantinos Charizanis, PhD, MBA Senior Director of Market Strategy and R&D Drey ColemanDirector Clinical Operations and Vendor Management Charlie Hoffmann, MBAVP Corporate Development and
Operations 25 years in life sciences fundraising, corporate finance, licensing and M&A transactions, and corporate development, Long time advisor to Ocularis/Nyxol. 14 years in medical research, product development, market research,
and biotech business development, with a focus on financial modeling, patents and genetics. 15 years in ophthalmic pharmaceutical research and development as sponsor and CRO with a focus on clinical operations, vendor management, pharma
regulations, and successful contract negotiations. 18 years in finance and accounting, for private and public companies, with a focus on life sciences. Held progressive roles including Controller, Director of Finance, and acting
CFO. Amy Rabourn, CPA VP Finance 4
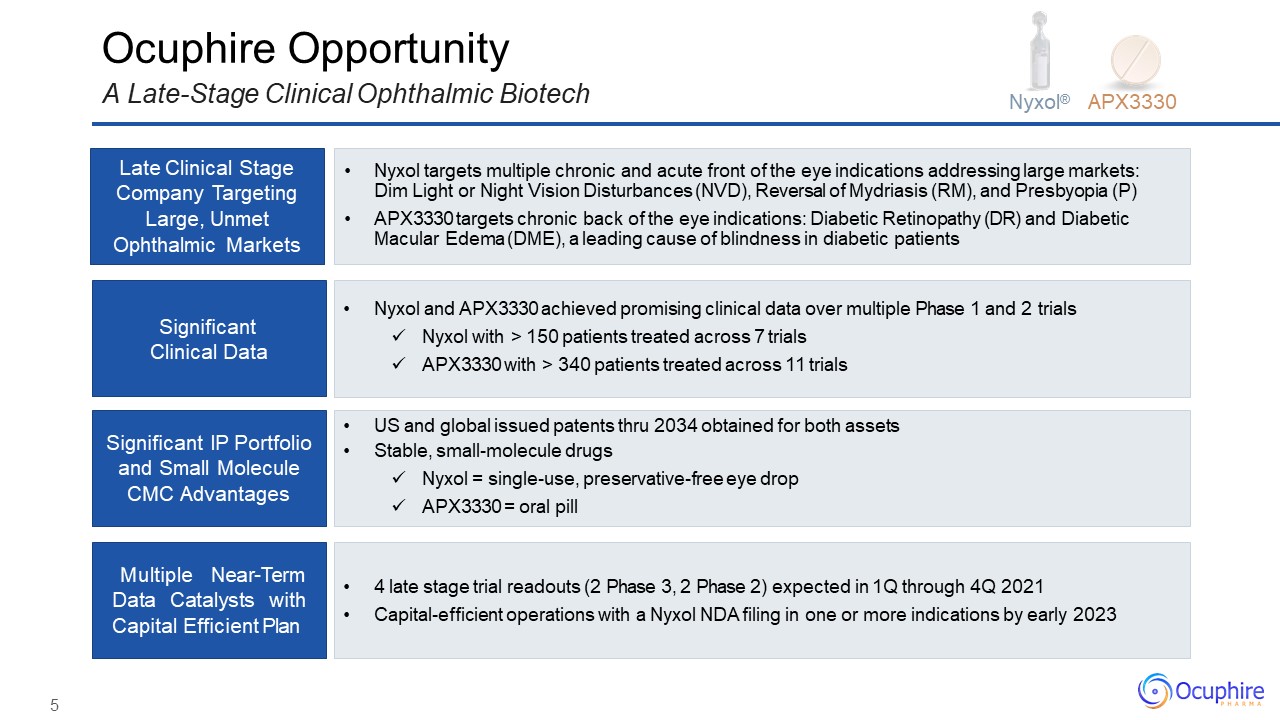
Late Clinical Stage Company Targeting Large, Unmet Ophthalmic Markets Significant Clinical
Data Significant IP Portfolio and Small Molecule CMC Advantages Multiple Near-Term Data Catalysts with Capital Efficient Plan Nyxol targets multiple chronic and acute front of the eye indications addressing large markets: Dim Light or
Night Vision Disturbances (NVD), Reversal of Mydriasis (RM), and Presbyopia (P)APX3330 targets chronic back of the eye indications: Diabetic Retinopathy (DR) and Diabetic Macular Edema (DME), a leading cause of blindness in diabetic
patients Nyxol and APX3330 achieved promising clinical data over multiple Phase 1 and 2 trialsNyxol with > 150 patients treated across 7 trialsAPX3330 with > 340 patients treated across 11 trials US and global issued patents thru
2034 obtained for both assetsStable, small-molecule drugsNyxol = single-use, preservative-free eye dropAPX3330 = oral pill 4 late stage trial readouts (2 Phase 3, 2 Phase 2) expected in 1Q through 4Q 2021Capital-efficient operations with
a Nyxol NDA filing in one or more indications by early 2023 Ocuphire OpportunityA Late-Stage Clinical Ophthalmic Biotech Nyxol® APX3330 5
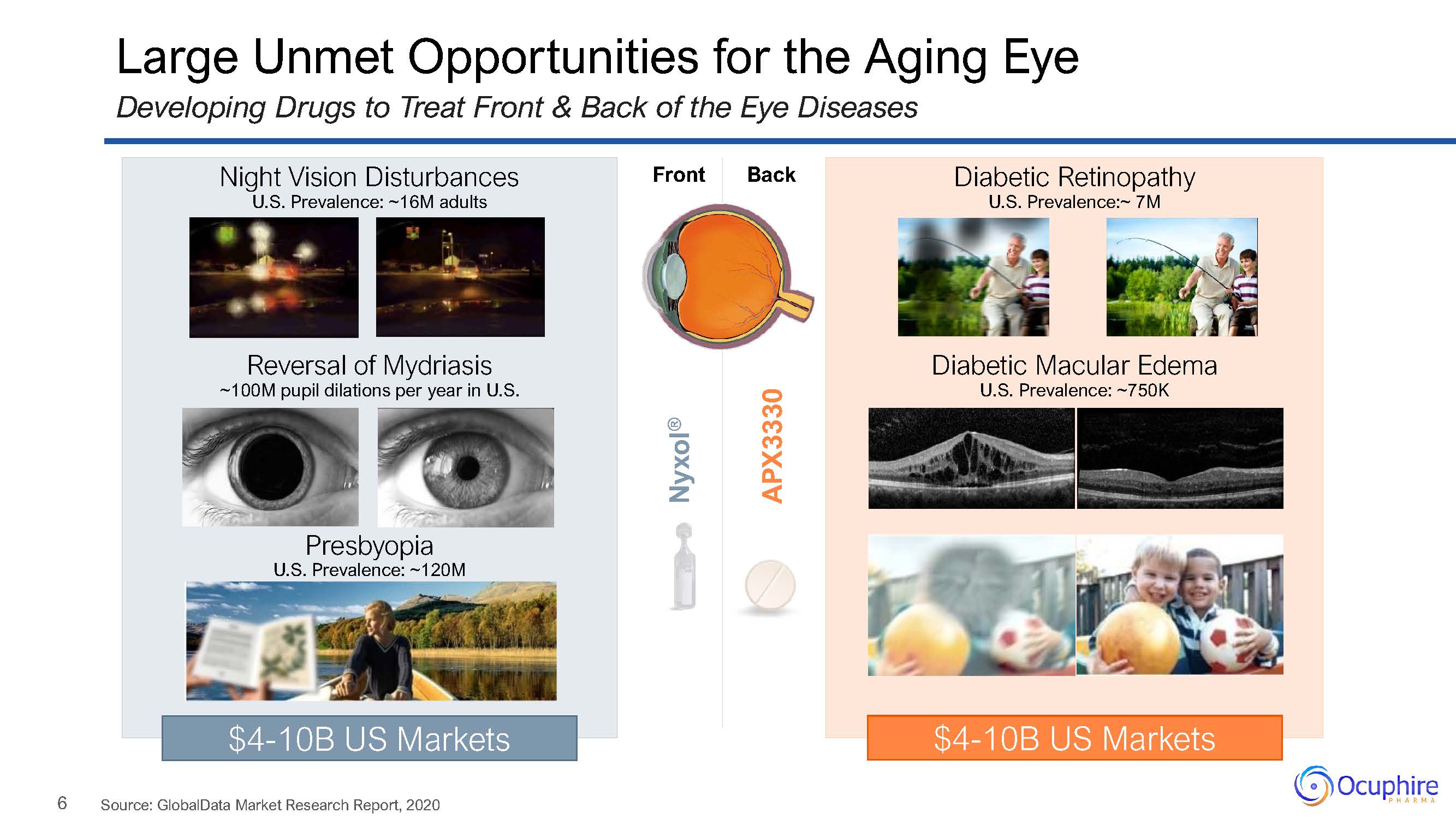
6 Large Unmet Opportunities for the Aging EyeDeveloping Drugs to Treat Front & Back of the
Eye Diseases Source: GlobalData Market Research Report, 2020 Night Vision DisturbancesU.S. Prevalence: ~16M adultsReversal of Mydriasis~100M pupil dilations per year in U.S.PresbyopiaU.S. Prevalence: ~120M $4-10B US
Markets Front Back Nyxol® APX3330 Diabetic RetinopathyU.S. Prevalence:~ 7MDiabetic Macular EdemaU.S. Prevalence: ~750K $4-10B US Markets
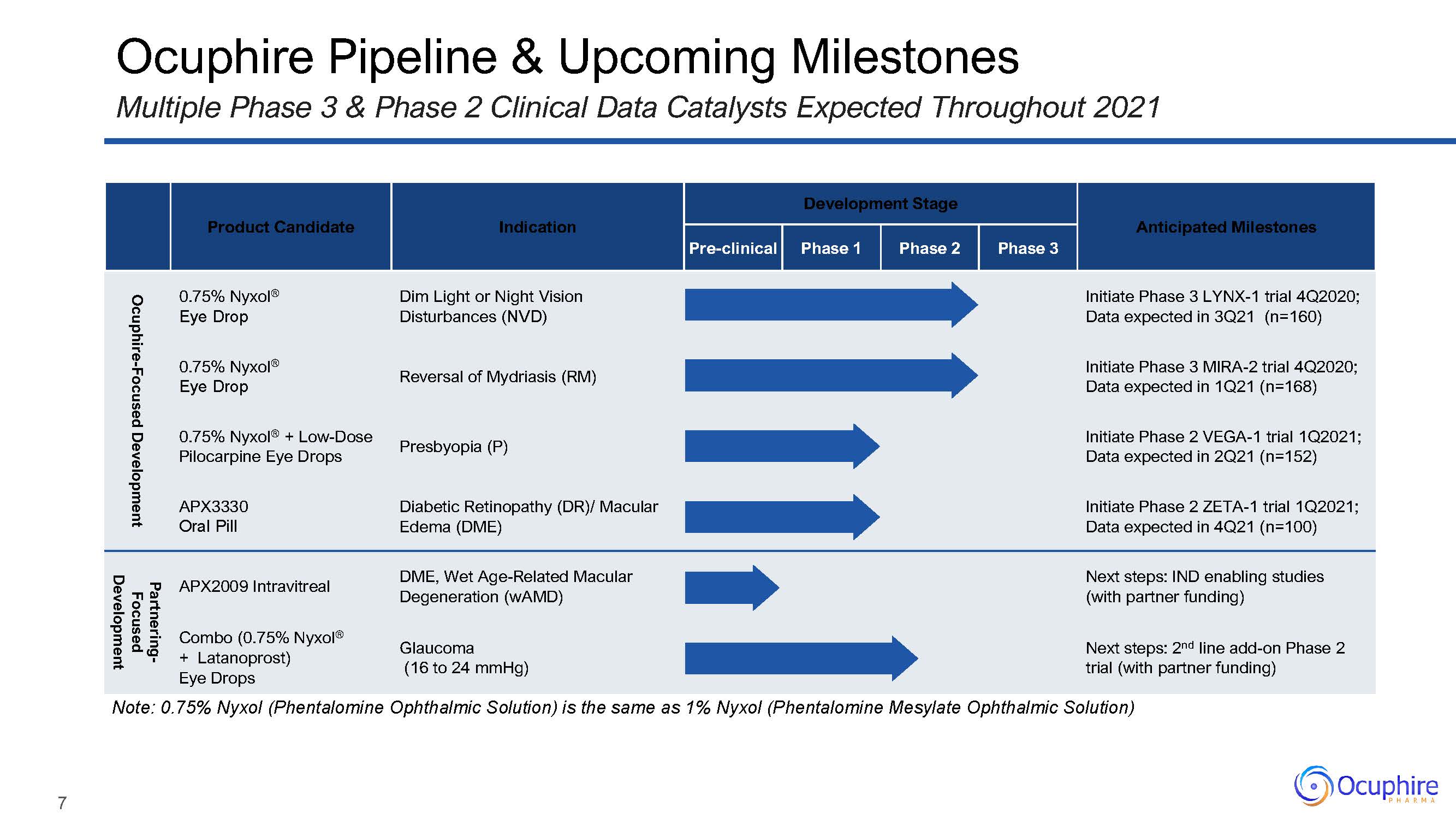
Product Candidate Indication Development Stage Anticipated
Milestones Pre-clinical Phase 1 Phase 2 Phase 3 Ocuphire-Focused Development 0.75% Nyxol®Eye Drop Dim Light or Night Vision Disturbances (NVD) Initiate Phase 3 LYNX-1 trial 4Q2020; Data expected in 3Q21
(n=160) 0.75% Nyxol®Eye Drop Reversal of Mydriasis (RM) Initiate Phase 3 MIRA-2 trial 4Q2020; Data expected in 1Q21 (n=168) 0.75% Nyxol® + Low-Dose Pilocarpine Eye Drops Presbyopia (P) Initiate Phase 2 VEGA-1
trial 1Q2021; Data expected in 2Q21 (n=152) APX3330Oral Pill Diabetic Retinopathy (DR)/ Macular Edema (DME) Initiate Phase 2 ZETA-1 trial 1Q2021; Data expected in 4Q21 (n=100) Partnering- Focused Development APX2009
Intravitreal DME, Wet Age-Related Macular Degeneration (wAMD) Next steps: IND enabling studies (with partner funding) Combo (0.75% Nyxol®+ Latanoprost) Eye Drops Glaucoma(16 to 24 mmHg) Next steps: 2nd line add-on
Phase 2 trial (with partner funding) Ocuphire Pipeline & Upcoming MilestonesMultiple Phase 3 & Phase 2 Clinical Data Catalysts Expected Throughout 2021 Note: 0.75% Nyxol (Phentalomine Ophthalmic Solution) is the same
as 1% Nyxol (Phentalomine Mesylate Ophthalmic Solution) 7
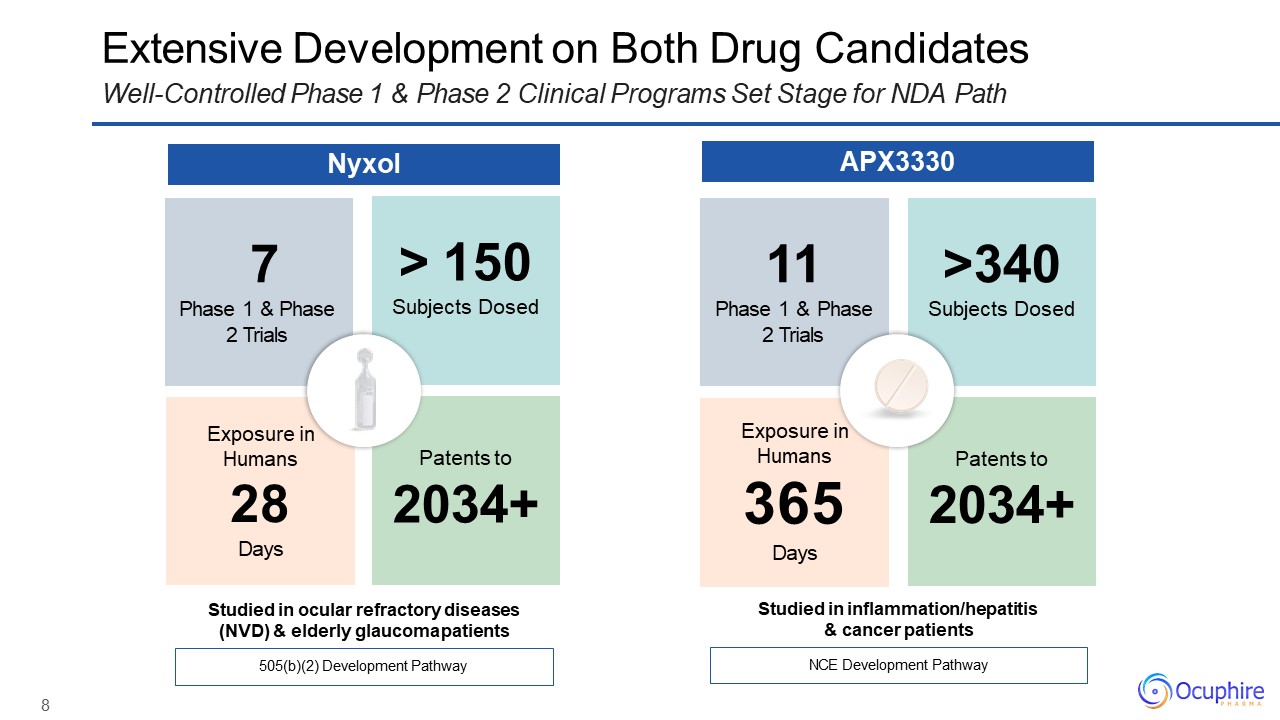
11 >340Phase 1 & Phase Subjects Dosed 2 Trials Exposure in
Humans365Days Patents to2034+ Extensive Development on Both Drug CandidatesWell-Controlled Phase 1 & Phase 2 Clinical Programs Set Stage for NDA Path NCE Development Pathway Studied in inflammation/hepatitis & cancer
patients 7Phase 1 & Phase 2 Trials > 150Subjects Dosed Exposure in Humans28Days Patents to2034+ 505(b)(2) Development Pathway 8 Studied in ocular refractory diseases (NVD) & elderly glaucoma
patients Nyxol APX3330
Nyxol® Phentolamine Mesylate NVD Night Vision Disturbances P
Presbyopia RM Reversal of Mydriasis 9
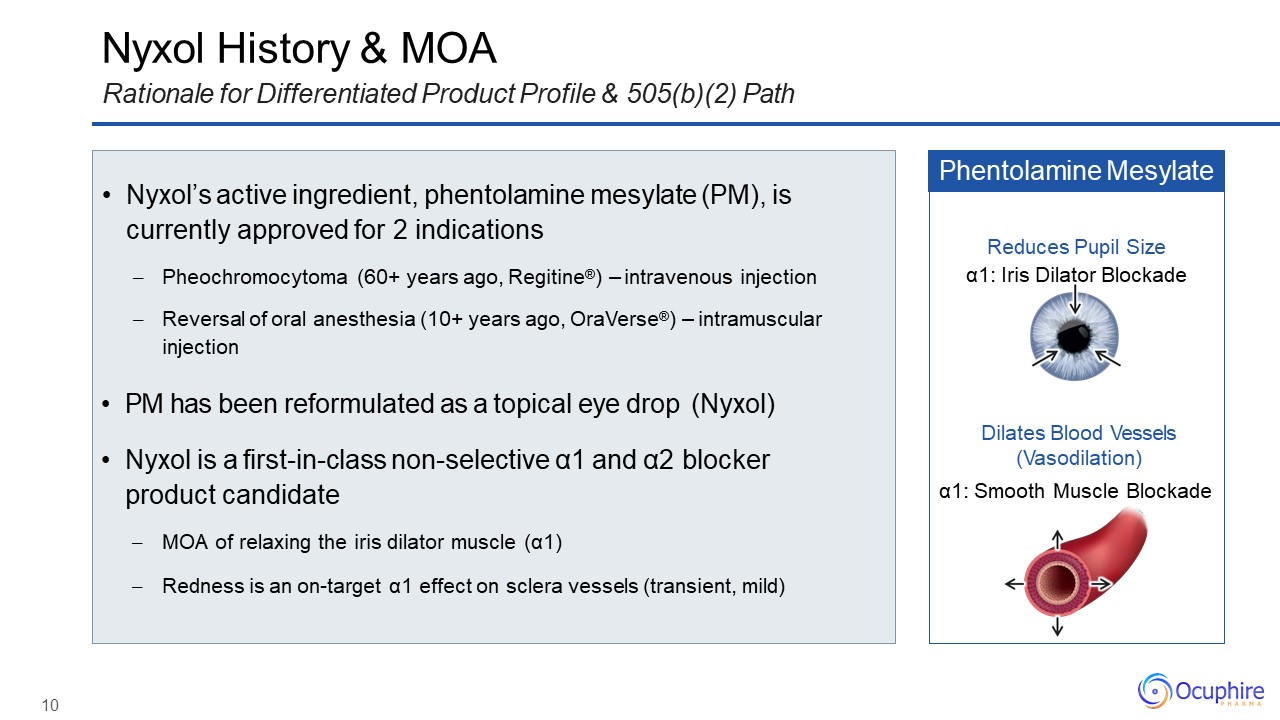
Nyxol History & MOARationale for Differentiated Product Profile & 505(b)(2)
Path Nyxol’s active ingredient, phentolamine mesylate (PM), iscurrently approved for 2 indicationsPheochromocytoma (60+ years ago, Regitine®) – intravenous injectionReversal of oral anesthesia (10+ years ago, OraVerse®) –
intramuscular injection PM has been reformulated as a topical eye drop (Nyxol)Nyxol is a first-in-class non-selective α1 and α2 blockerproduct candidateMOA of relaxing the iris dilator muscle (α1)Redness is an on-target α1 effect on
sclera vessels (transient, mild) Phentolamine Mesylate Dilates Blood Vessels (Vasodilation)α1: Smooth Muscle Blockade Reduces Pupil Sizeα1: Iris Dilator Blockade 10
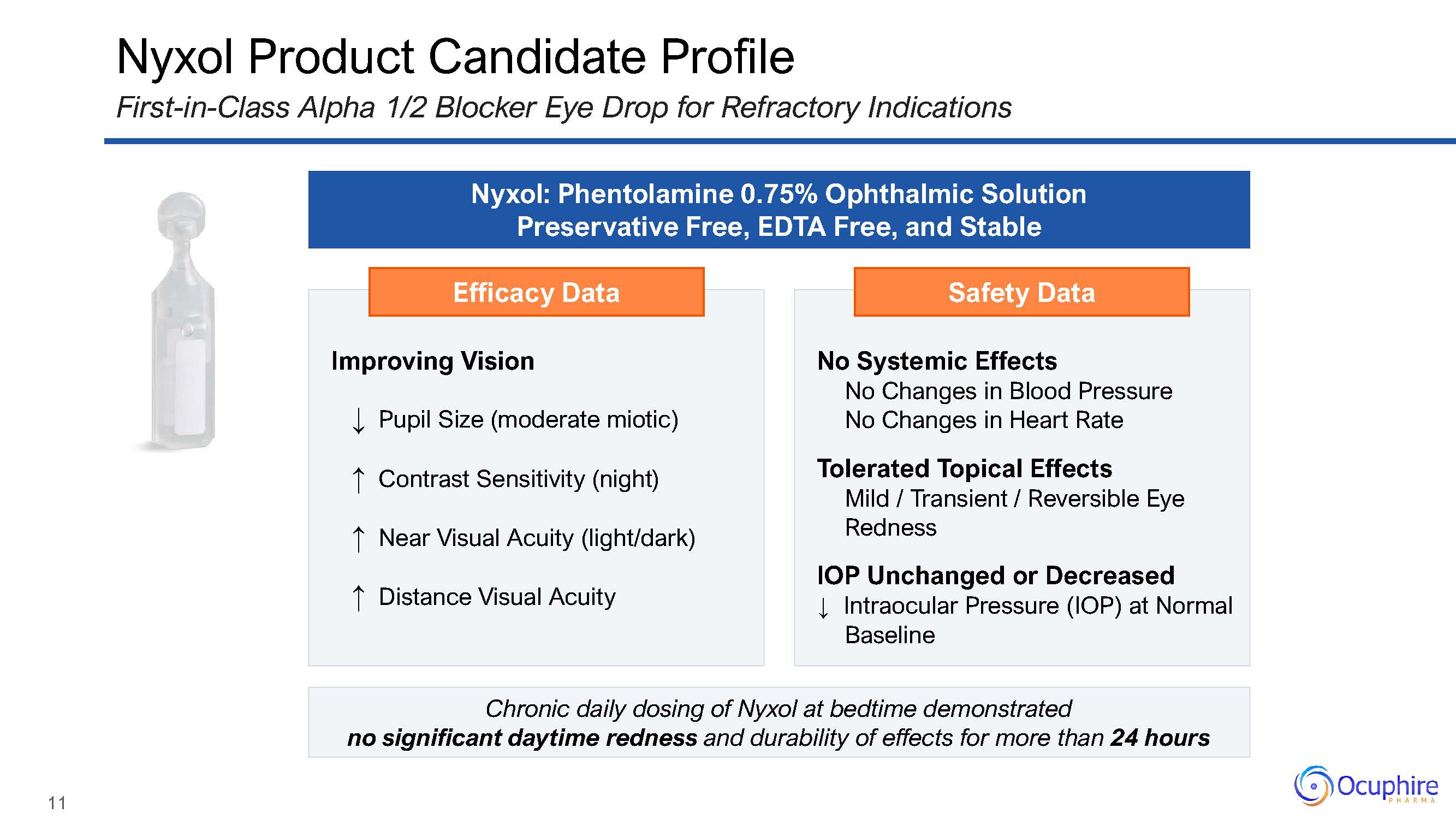
Efficacy Data Improving Vision↓ Pupil Size (moderate miotic)↑ Contrast Sensitivity
(night)↑ Near Visual Acuity (light/dark)↑ Distance Visual Acuity Safety Data No Systemic EffectsNo Changes in Blood Pressure No Changes in Heart RateTolerated Topical EffectsMild / Transient / Reversible Eye RednessIOP
Unchanged or Decreased↓ Intraocular Pressure (IOP) at Normal Baseline Nyxol Product Candidate ProfileFirst-in-Class Alpha 1/2 Blocker Eye Drop for Refractory Indications Nyxol: Phentolamine 0.75% Ophthalmic Solution Preservative
Free, EDTA Free, and Stable Chronic daily dosing of Nyxol at bedtime demonstratedno significant daytime redness and durability of effects for more than 24 hours 11
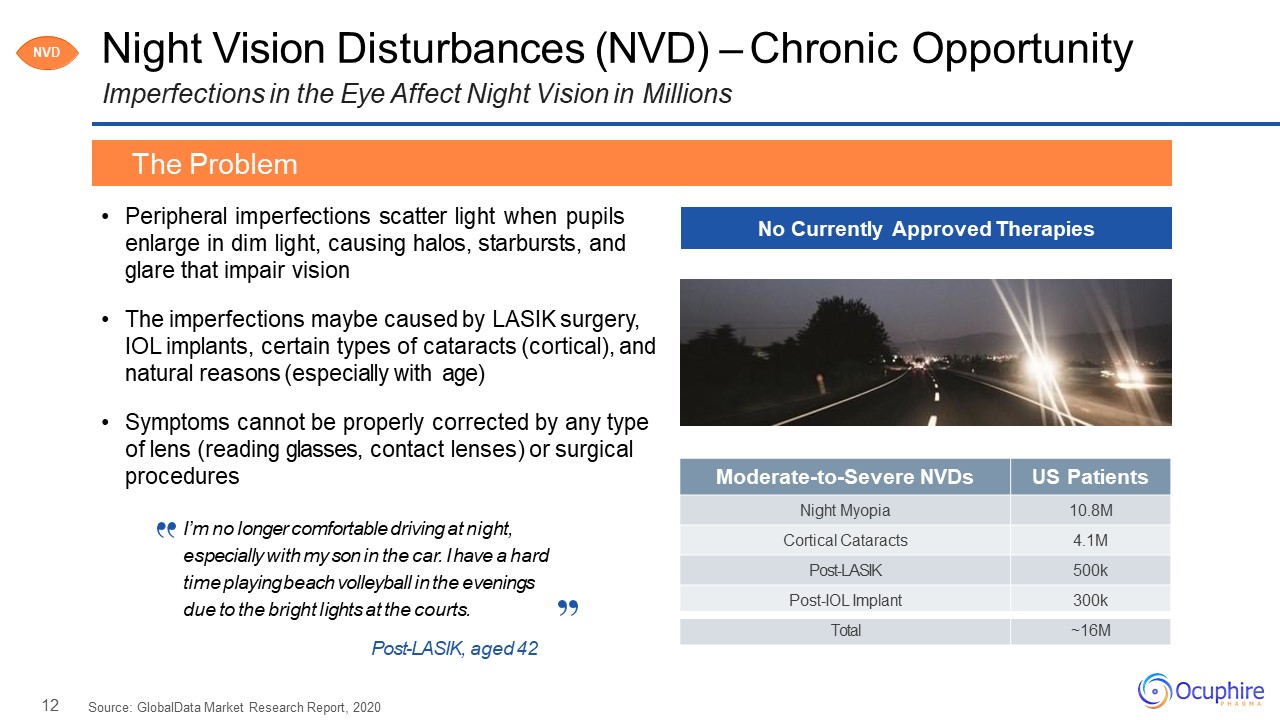
12 Moderate-to-Severe NVDs US Patients Night Myopia 10.8M Cortical
Cataracts 4.1M Post-LASIK 500k Post-IOL Implant 300k Total ~16M Night Vision Disturbances (NVD) – Chronic OpportunityImperfections in the Eye Affect Night Vision in Millions Peripheral imperfections scatter light when pupils
enlarge in dim light, causing halos, starbursts, and glare that impair visionThe imperfections maybe caused by LASIK surgery, IOL implants, certain types of cataracts (cortical), and natural reasons (especially with age)Symptoms cannot be
properly corrected by any type of lens (reading glasses, contact lenses) or surgical procedures Source: GlobalData Market Research Report, 2020 The Problem No Currently Approved Therapies NVD I’m no longer comfortable driving at
night, especially with my son in the car. I have a hard time playing beach volleyball in the evenings due to the bright lights at the courts.Post-LASIK, aged 42
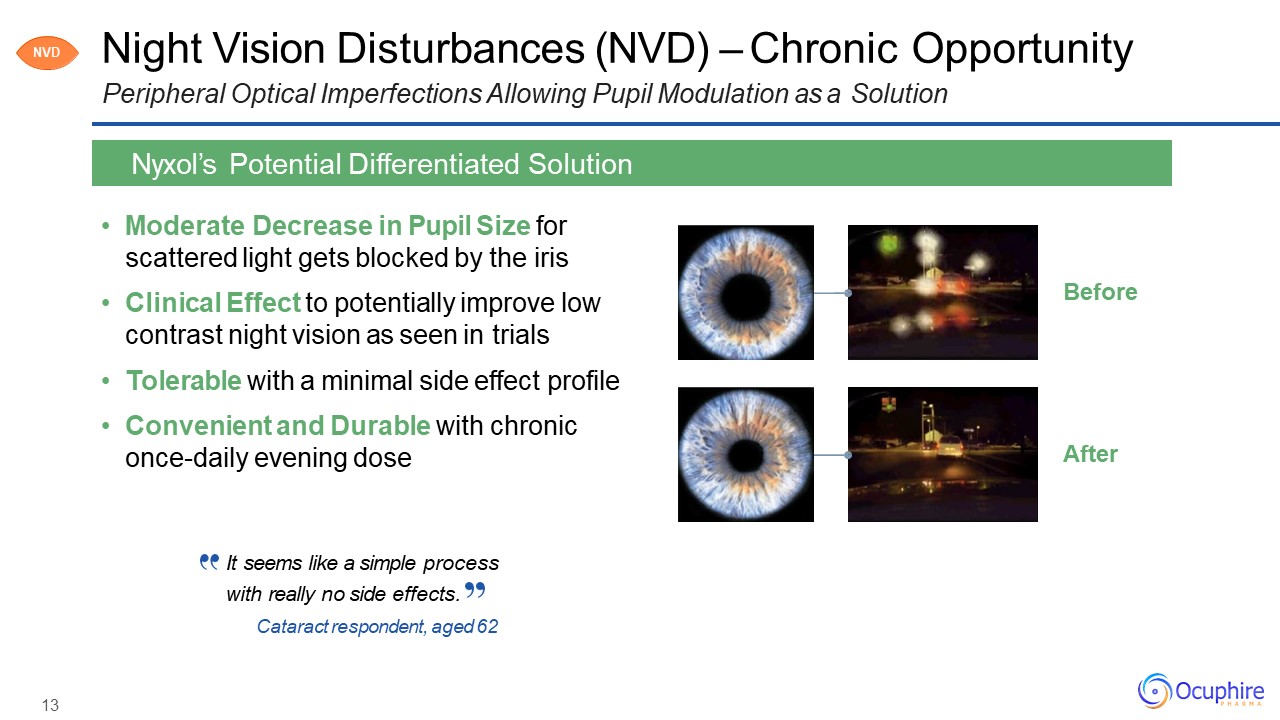
Night Vision Disturbances (NVD) – Chronic OpportunityPeripheral Optical Imperfections Allowing
Pupil Modulation as a Solution Moderate Decrease in Pupil Size for scattered light gets blocked by the irisClinical Effect to potentially improve low contrast night vision as seen in trialsTolerable with a minimal side effect
profileConvenient and Durable with chronic once-daily evening dose Nyxol’s Potential Differentiated Solution After Before NVD It seems like a simple processwith really no side effects.Cataract respondent, aged
62 13
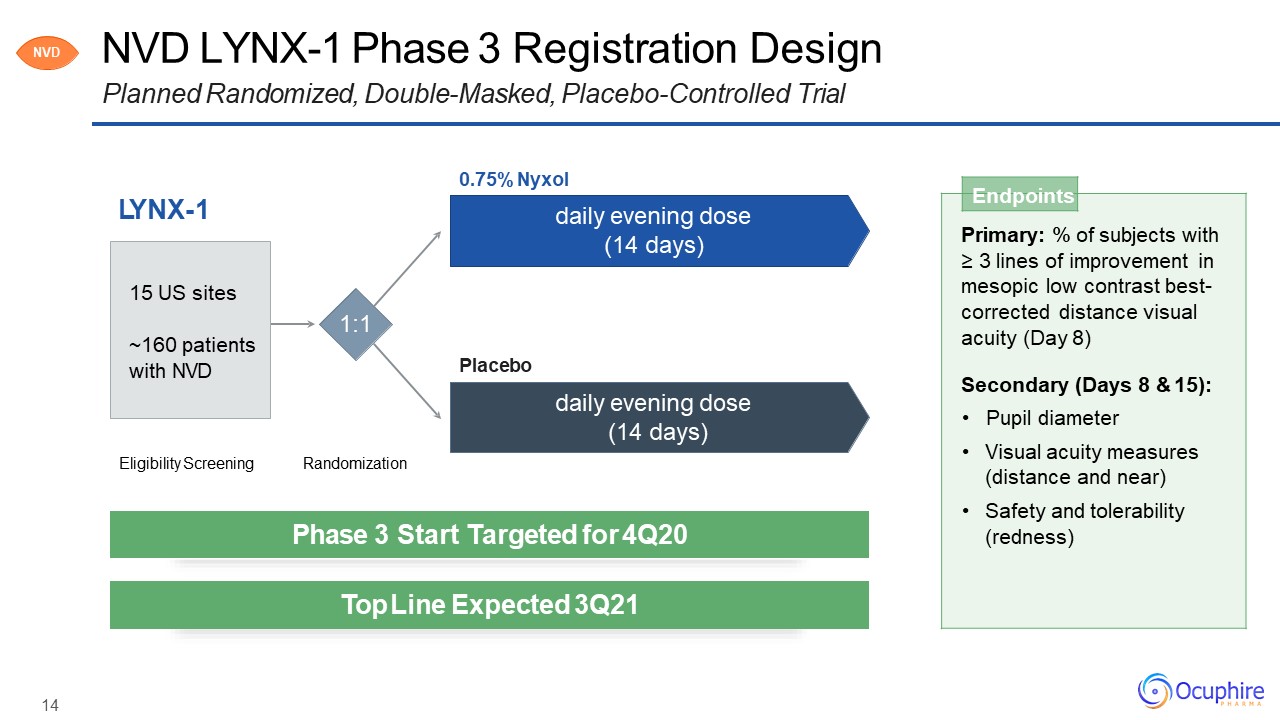
NVD LYNX-1 Phase 3 Registration DesignPlanned Randomized, Double-Masked, Placebo-Controlled
Trial LYNX-1 Endpoints Primary: % of subjects with≥ 3 lines of improvement inmesopic low contrast best- corrected distance visual acuity (Day 8)Secondary (Days 8 & 15):Pupil diameterVisual acuity measures (distance and
near)Safety and tolerability (redness) Eligibility Screening Randomization 1:1 0.75% Nyxoldaily evening dose (14 days) daily evening dose (14 days) Placebo 15 US sites~160 patients with NVD NVD Phase 3 Start
Targeted for 4Q20 Top Line Expected 3Q21 14
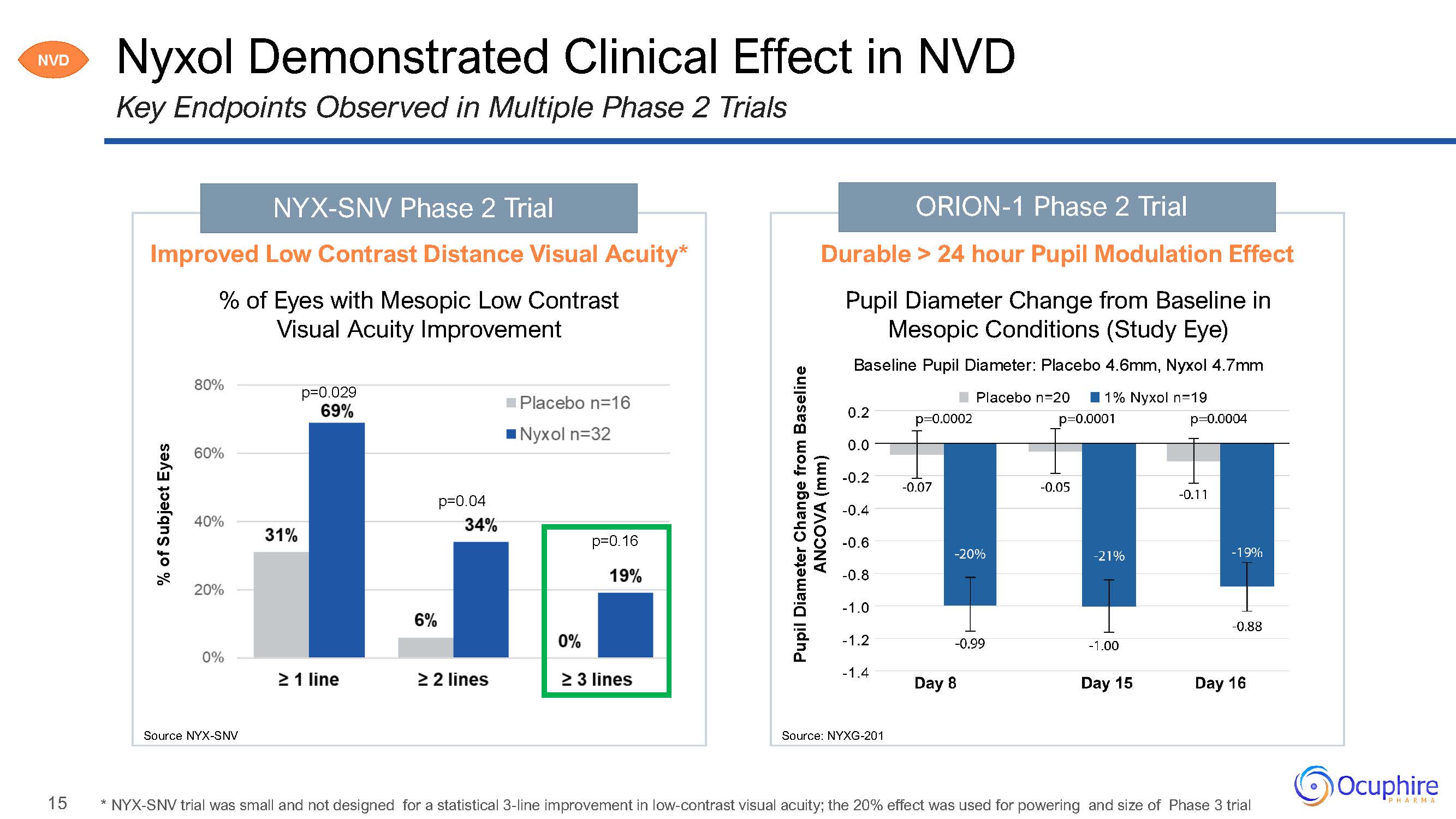
15 ORION-1 Phase 2 Trial Nyxol Demonstrated Clinical Effect in NVDKey Endpoints Observed
in Multiple Phase 2 Trials Source: NYXG-201 % of Subject Eyes NYX-SNV Phase 2 Trial Improved Low Contrast Distance Visual Acuity*% of Eyes with Mesopic Low Contrast Visual Acuity Improvementp=0.029p=0.04p=0.16Source
NYX-SNV Pupil Diameter Change from Baseline ANCOVA (mm) Durable > 24 hour Pupil Modulation EffectPupil Diameter Change from Baseline in Mesopic Conditions (Study Eye)Baseline Pupil Diameter: Placebo 4.6mm, Nyxol
4.7mm NVD * NYX-SNV trial was small and not designed for a statistical 3-line improvement in low-contrast visual acuity; the 20% effect was used for powering and size of Phase 3 trial
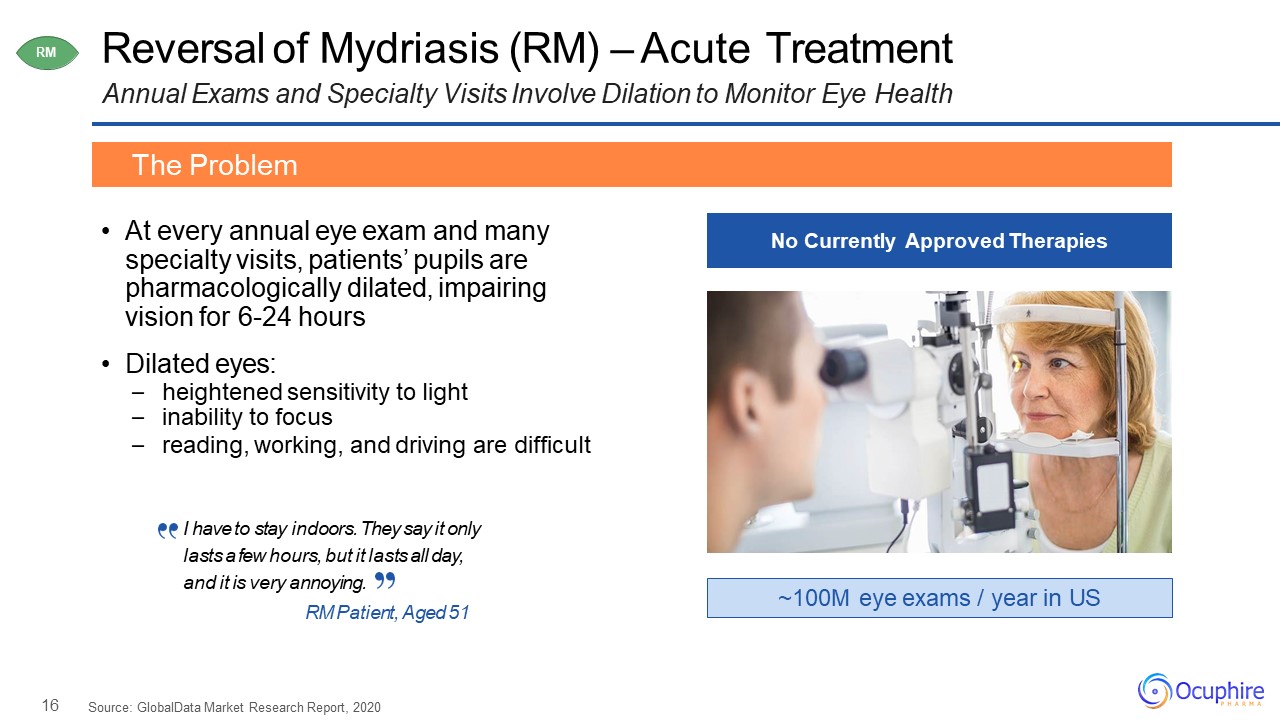
16 Reversal of Mydriasis (RM) – Acute TreatmentAnnual Exams and Specialty Visits Involve
Dilation to Monitor Eye Health At every annual eye exam and many specialty visits, patients’ pupils are pharmacologically dilated, impairing vision for 6-24 hoursDilated eyes:heightened sensitivity to lightinability to focusreading,
working, and driving are difficult The Problem ~100M eye exams / year in US No Currently Approved Therapies RM Source: GlobalData Market Research Report, 2020 I have to stay indoors. They say it only lasts a few hours, but it
lasts all day, and it is very annoying.RM Patient, Aged 51
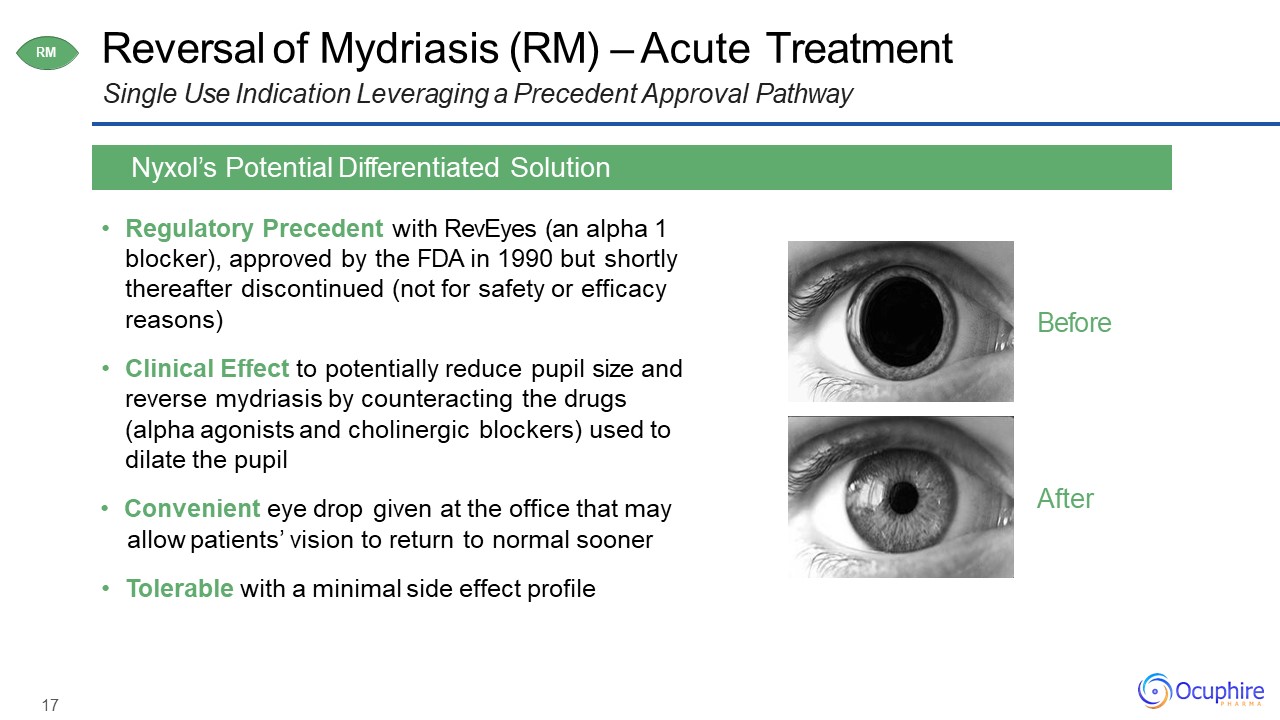
17 Reversal of Mydriasis (RM) – Acute TreatmentSingle Use Indication Leveraging a Precedent
Approval Pathway Regulatory Precedent with RevEyes (an alpha 1 blocker), approved by the FDA in 1990 but shortly thereafter discontinued (not for safety or efficacy reasons)Clinical Effect to potentially reduce pupil size and reverse
mydriasis by counteracting the drugs (alpha agonists and cholinergic blockers) used to dilate the pupilConvenient eye drop given at the office that mayallow patients’ vision to return to normal soonerTolerable with a minimal side effect
profile Nyxol’s Potential Differentiated Solution RM After Before
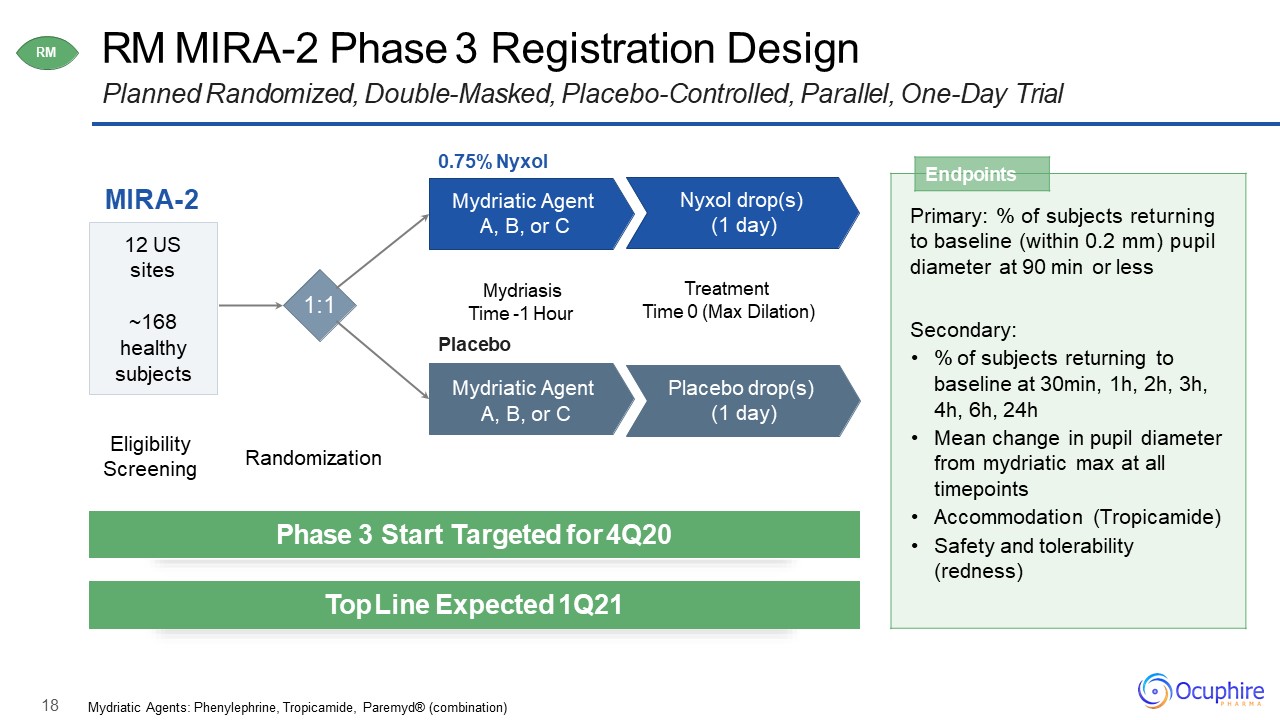
18 RM MIRA-2 Phase 3 Registration DesignPlanned Randomized, Double-Masked, Placebo-Controlled,
Parallel, One-Day Trial MIRA-2 0.75% NyxolMydriatic Agent A, B, or C 12 USsites~168healthy subjects Nyxol drop(s) (1 day) Mydriasis Time -1 HourPlaceboMydriatic Agent A, B, or C Placebo drop(s) (1 day) TreatmentTime
0 (Max Dilation) RM Endpoints Primary: % of subjects returning to baseline (within 0.2 mm) pupil diameter at 90 min or lessSecondary:% of subjects returning to baseline at 30min, 1h, 2h, 3h, 4h, 6h, 24hMean change in
pupil diameter from mydriatic max at all timepointsAccommodation (Tropicamide)Safety and tolerability (redness) Phase 3 Start Targeted for 4Q20 Top Line Expected 1Q21 Eligibility Screening Randomization Mydriatic Agents:
Phenylephrine, Tropicamide, Paremyd® (combination) 1:1
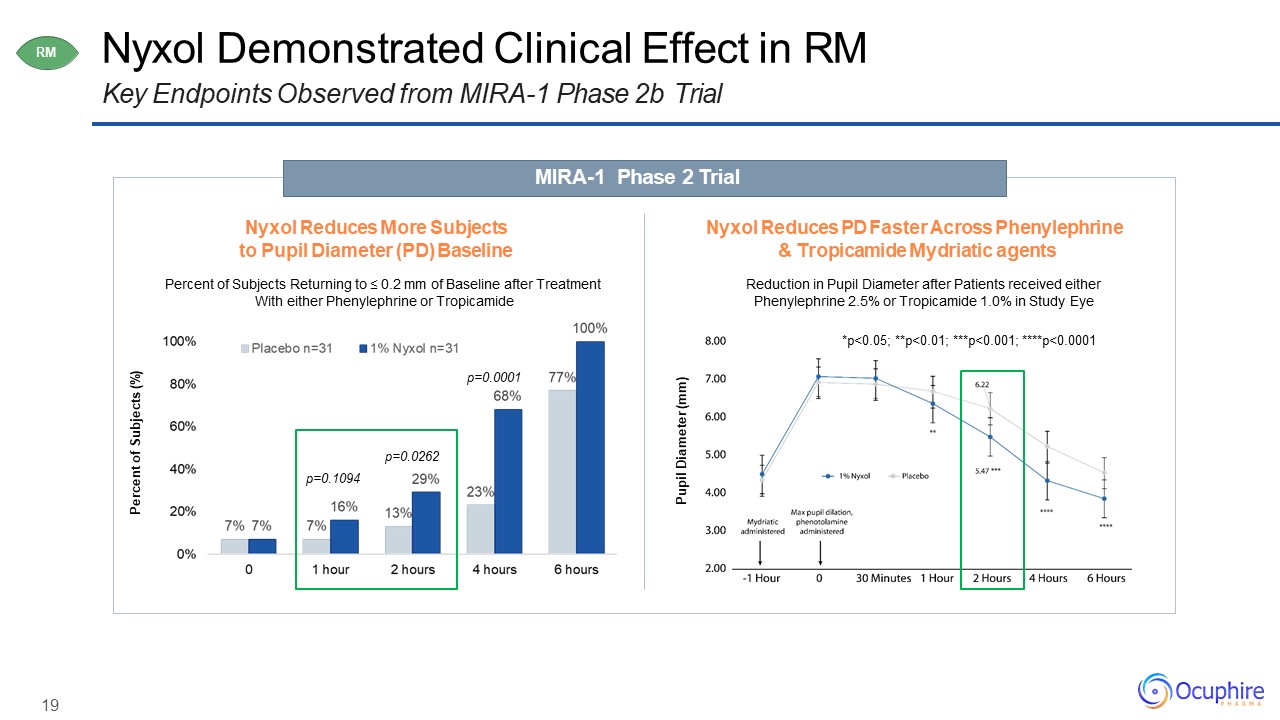
19 Nyxol Demonstrated Clinical Effect in RMKey Endpoints Observed from MIRA-1 Phase 2b
Trial MIRA-1 Phase 2 Trial RM Nyxol Reduces More Subjects to Pupil Diameter (PD) BaselinePercent of Subjects Returning to ≤ 0.2 mm of Baseline after Treatment With either Phenylephrine or Tropicamide Nyxol Reduces PD Faster
Across Phenylephrine & Tropicamide Mydriatic agentsReduction in Pupil Diameter after Patients received either Phenylephrine 2.5% or Tropicamide 1.0% in Study Eye Percent of Subjects (%) p=0.0262p=0.1094 p=0.0001 Pupil Diameter
(mm) *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001
20 Presbyopia – Chronic OpportunityAging Population Drives Demand for Alternatives to Reading
Glasses Lens loses ability to change shape when viewing objects up close as we ageDependence on reading glasses for intermittent and prolonged useGrowing need for therapies that improve, rather than hinder, quality of life Source:
GlobalData Market Research Report, 2020 The Problem P 120 MPatients No Currently Approved Therapies Effectively everyone over 40 will have the problems with reading.Physician KOL
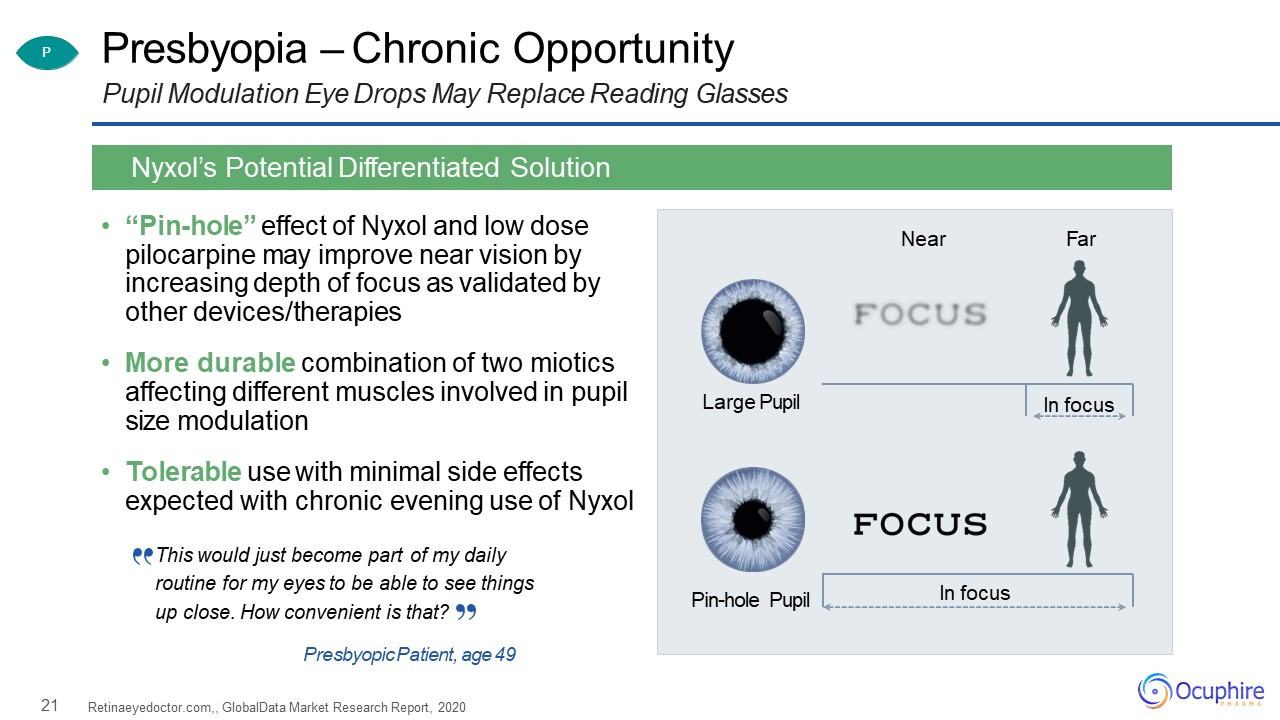
21 Presbyopia – Chronic OpportunityPupil Modulation Eye Drops May Replace Reading
Glasses “Pin-hole” effect of Nyxol and low dose pilocarpine may improve near vision by increasing depth of focus as validated by other devices/therapiesMore durable combination of two miotics affecting different muscles involved in pupil
size modulationTolerable use with minimal side effects expected with chronic evening use of Nyxol Retinaeyedoctor.com,, GlobalData Market Research Report, 2020 Nyxol’s Potential Differentiated Solution Large Pupil Pin-hole
Pupil Near Far In focus In focus P This would just become part of my daily routine for my eyes to be able to see things up close. How convenient is that?Presbyopic Patient, age 49
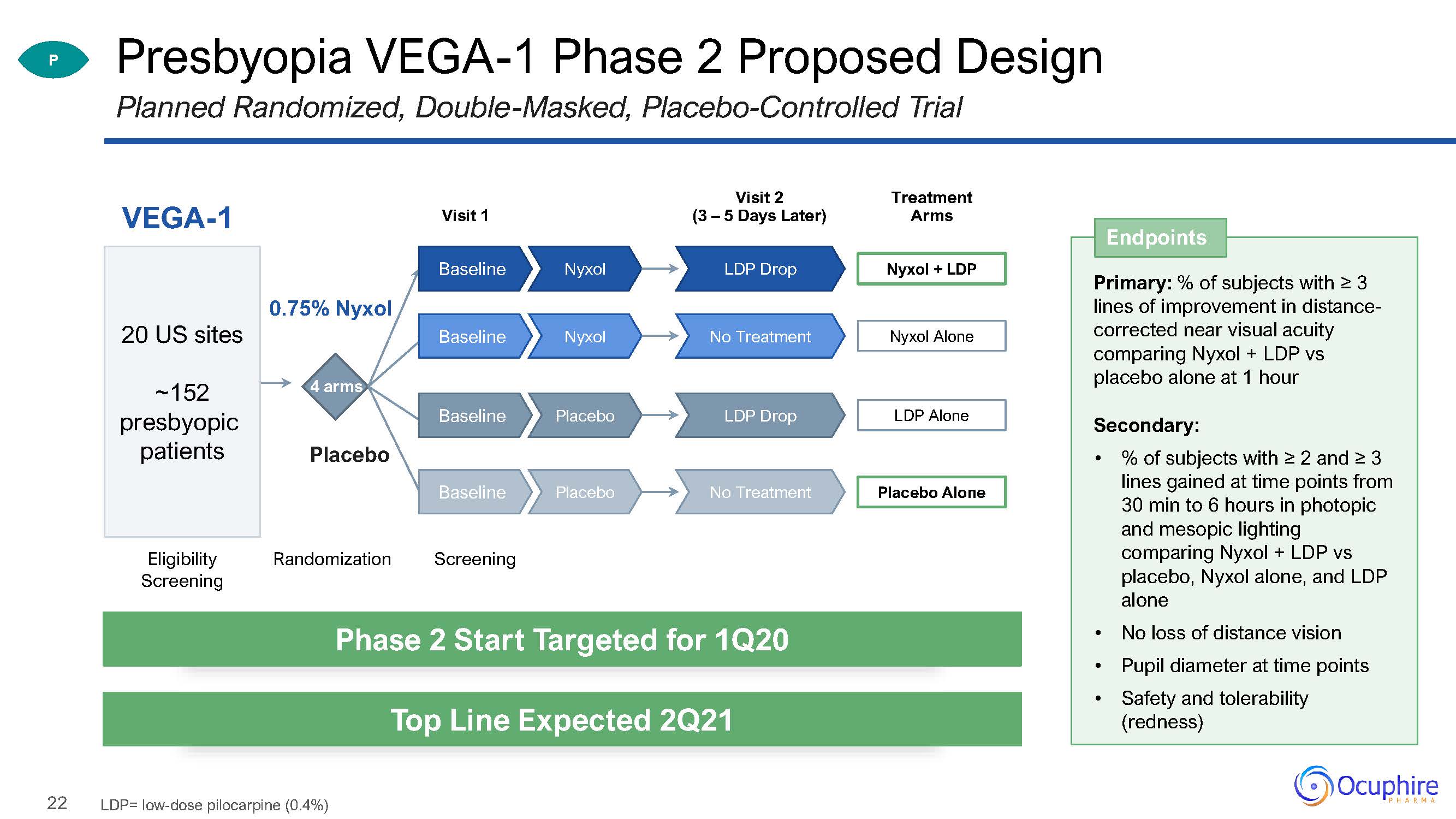
22 Presbyopia VEGA-1 Phase 2 Proposed DesignPlanned Randomized, Double-Masked,
Placebo-Controlled Trial Visit 1 VEGA-1 Eligibility Screening Randomization 4 arms 0.75% Nyxol Placebo 20 US sites~152presbyopic patients Visit 2(3 – 5 Days Later) Screening P Endpoints Primary: % of
subjects with ≥ 3 lines of improvement in distance- corrected near visual acuity comparing Nyxol + LDP vs placebo alone at 1 hourSecondary:% of subjects with ≥ 2 and ≥ 3 lines gained at time points from 30 min to 6 hours in photopic and
mesopic lighting comparing Nyxol + LDP vs placebo, Nyxol alone, and LDP aloneNo loss of distance visionPupil diameter at time pointsSafety and tolerability (redness) Phase 2 Start Targeted for 1Q20 Top Line Expected 2Q21 LDP=
low-dose pilocarpine (0.4%) Treatment Arms Nyxol + LDP LDP Drop Nyxol Baseline Nyxol Alone No Treatment Nyxol Baseline LDP Alone LDP Drop Placebo Baseline Placebo
Alone No Treatment Placebo Baseline
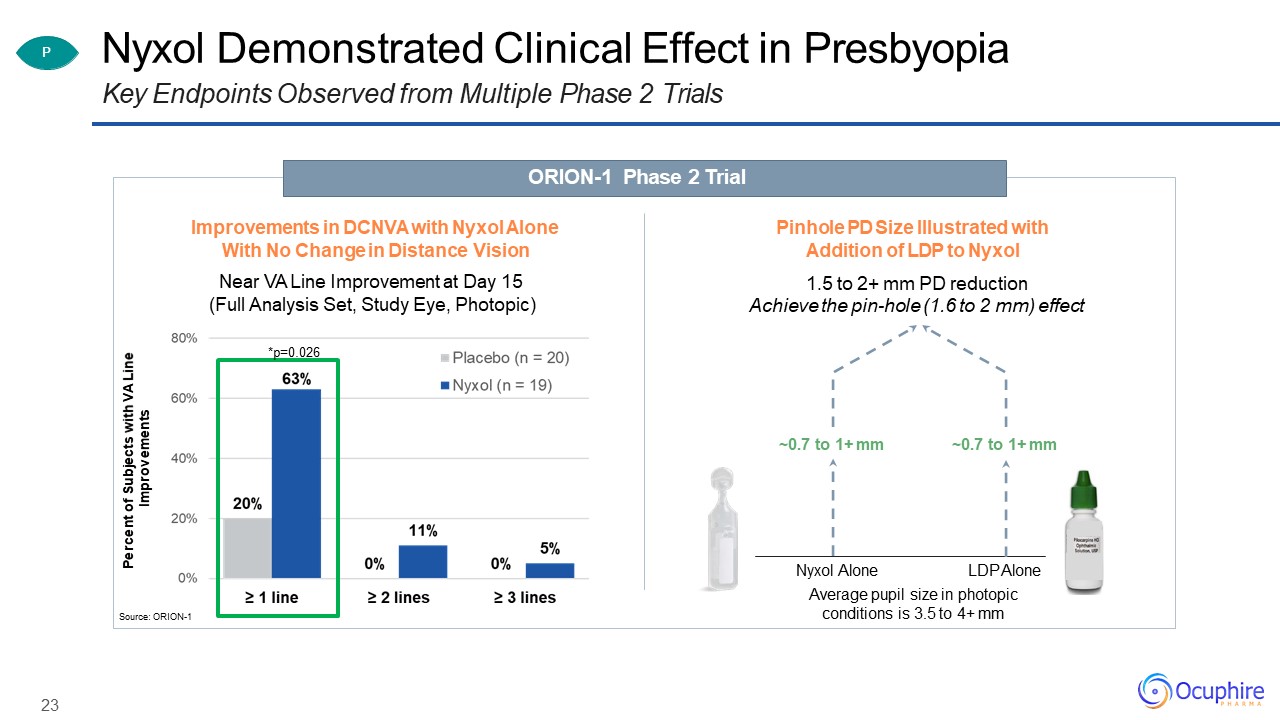
Nyxol Demonstrated Clinical Effect in PresbyopiaKey Endpoints Observed from Multiple Phase 2
Trials Source: ORION-1 Nyxol Alone LDP AloneAverage pupil size in photopic conditions is 3.5 to 4+ mm ~0.7 to 1+ mm P ORION-1 Phase 2 Trial Pinhole PD Size Illustrated with Addition of LDP to Nyxol1.5 to 2+ mm PD
reductionAchieve the pin-hole (1.6 to 2 mm) effect Percent of Subjects with VA Line Improvements 23 Improvements in DCNVA with Nyxol Alone With No Change in Distance VisionNear VA Line Improvement at Day 15 (Full Analysis Set,
Study Eye, Photopic)*p=0.026 ~0.7 to 1+ mm

APX3330 APX3330 DR Diabetic Retinopathy DME Diabetic Macular
Edema wAMD Wet Age-Related Macular Degeneration 24
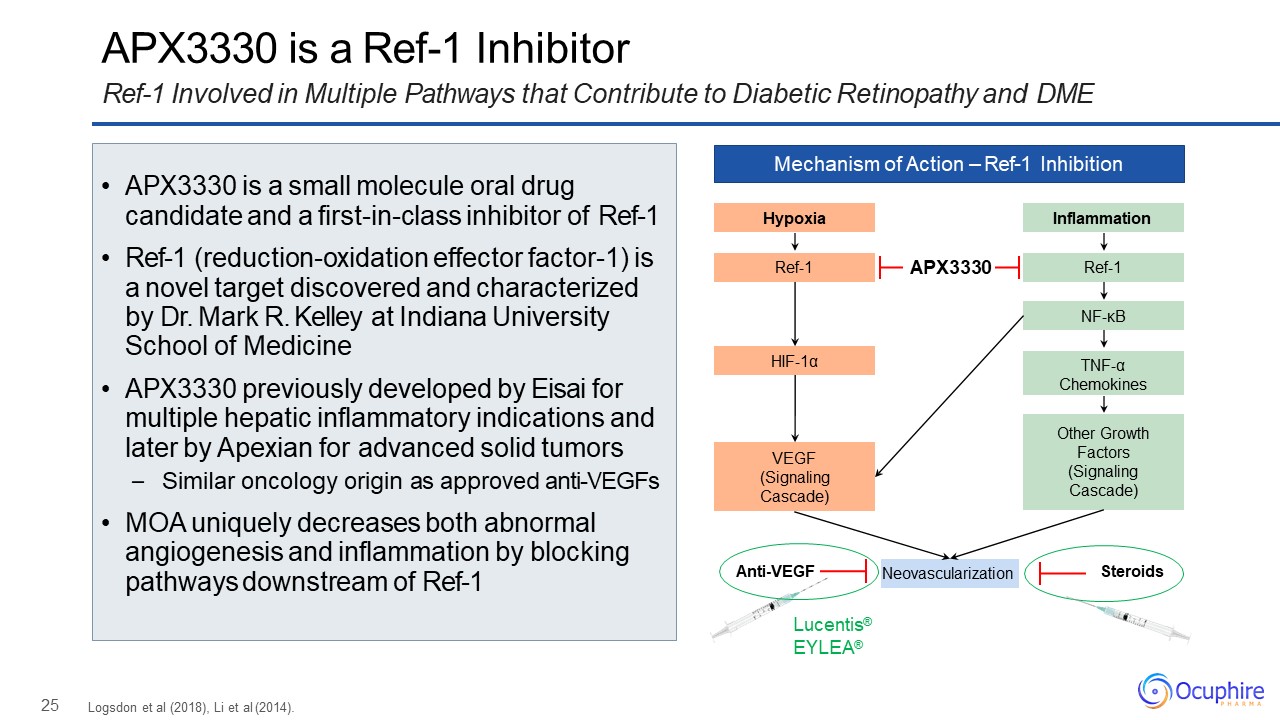
25 APX3330 is a Ref-1 InhibitorRef-1 Involved in Multiple Pathways that Contribute to Diabetic
Retinopathy and DME Mechanism of Action – Ref-1 Inhibition Hypoxia Ref-1 HIF-1α VEGF(Signaling Cascade) Inflammation Ref-1 NF-κB Other Growth Factors (Signaling
Cascade) TNF-αChemokines Neovascularization Lucentis® EYLEA® Anti-VEGF Steroids APX3330 Logsdon et al (2018), Li et al (2014). APX3330 is a small molecule oral drug candidate and a
first-in-class inhibitor of Ref-1Ref-1 (reduction-oxidation effector factor-1) is a novel target discovered and characterized by Dr. Mark R. Kelley at Indiana University School of MedicineAPX3330 previously developed by Eisai for multiple
hepatic inflammatory indications and later by Apexian for advanced solid tumors– Similar oncology origin as approved anti-VEGFsMOA uniquely decreases both abnormal angiogenesis and inflammation by blocking pathways downstream of Ref-1
26 APX3330 Product Candidate ProfileFirst-in-Class Ref-1 Inhibitor Phase 2 Ready for Retina
Diabetic Indications Expected Efficacy Data Improving Eye Health in Diabetics↓ Inflammation↓ Hypoxia Signaling↓ Abnormal AngiogenesisEnhance Compliance & Exposure Oral pill may reduce the burden of frequent anti-VEGF
injections Safety Data Few Systemic Adverse EffectsMild Gastrointestinal (diarrhea)Mild Skin Rash (Reversible)Lack of Significant Acute Neurologic, Cardiovascular, Liver, or Pulmonary toxicityNo Topical EffectsNo observed
ocular AEs APX3330: 600mg Oral Dose (120mg or 300mg tablets) Twice a day dosing of APX3330 anticipated to provide steady state effectiveness with a tolerable chronic safety profile
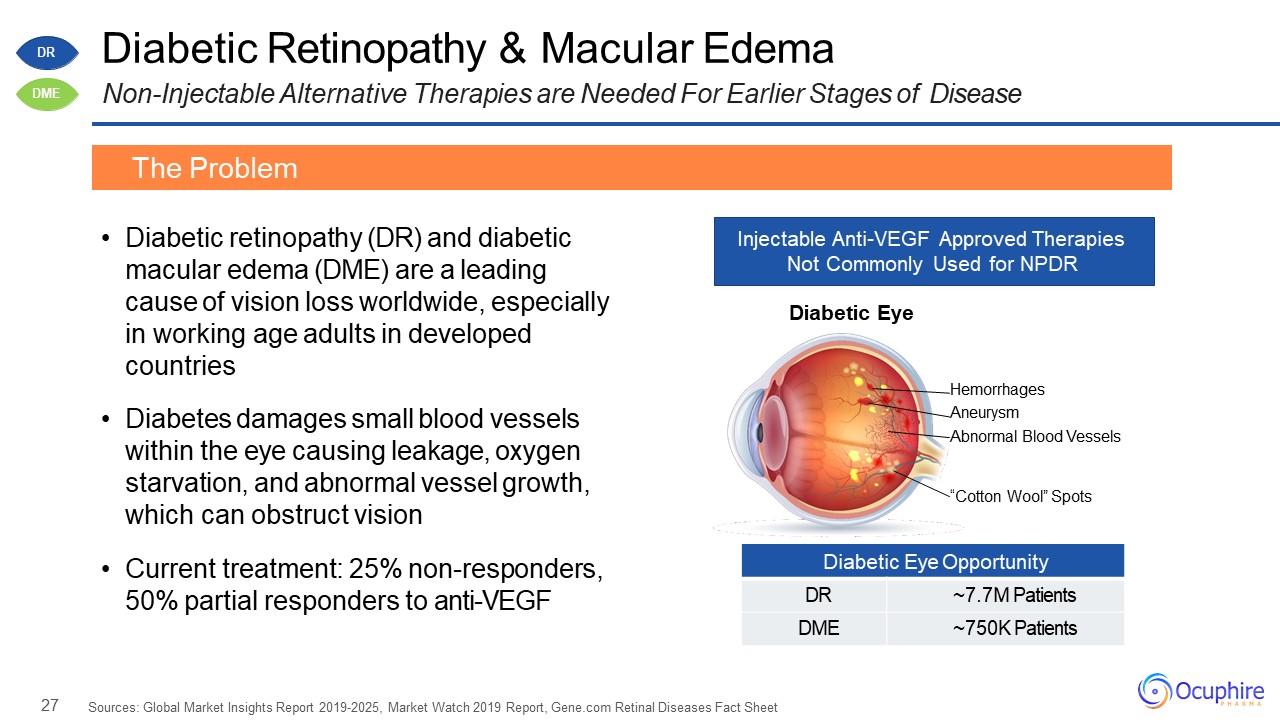
27 Diabetic Eye Opportunity DR ~7.7M Patients DME ~750K Patients Diabetic Retinopathy
& Macular EdemaNon-Injectable Alternative Therapies are Needed For Earlier Stages of Disease Diabetic retinopathy (DR) and diabetic macular edema (DME) are a leading cause of vision loss worldwide, especially in working age adults in
developed countriesDiabetes damages small blood vessels within the eye causing leakage, oxygen starvation, and abnormal vessel growth, which can obstruct visionCurrent treatment: 25% non-responders, 50% partial responders to
anti-VEGF Sources: Global Market Insights Report 2019-2025, Market Watch 2019 Report, Gene.com Retinal Diseases Fact Sheet The Problem Injectable Anti-VEGF Approved Therapies Not Commonly Used for NPDR “Cotton Wool” Spots Diabetic
Eye Hemorrhages AneurysmAbnormal Blood Vessels DR DME
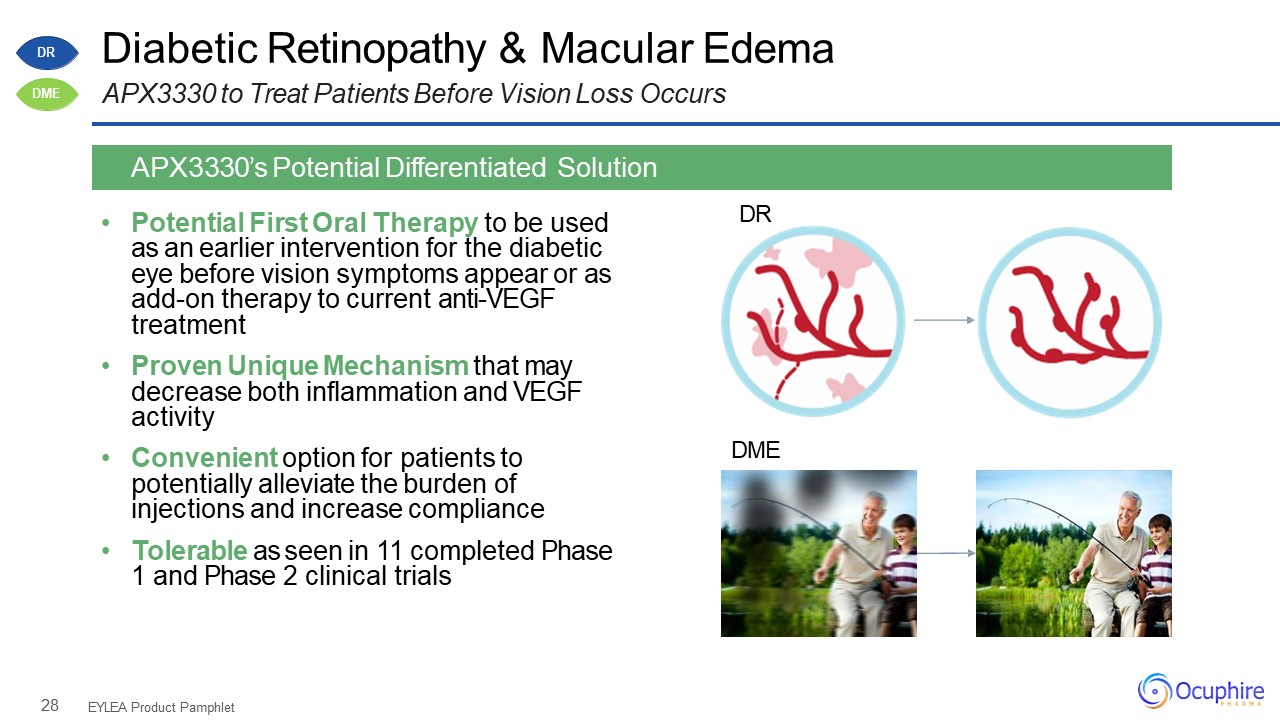
28 Diabetic Retinopathy & Macular EdemaAPX3330 to Treat Patients Before Vision Loss
Occurs Potential First Oral Therapy to be used as an earlier intervention for the diabetic eye before vision symptoms appear or as add-on therapy to current anti-VEGF treatmentProven Unique Mechanism that may decrease both inflammation
and VEGF activityConvenient option for patients to potentially alleviate the burden of injections and increase complianceTolerable as seen in 11 completed Phase 1 and Phase 2 clinical trials EYLEA Product Pamphlet APX3330’s Potential
Differentiated Solution DR DME DR DME
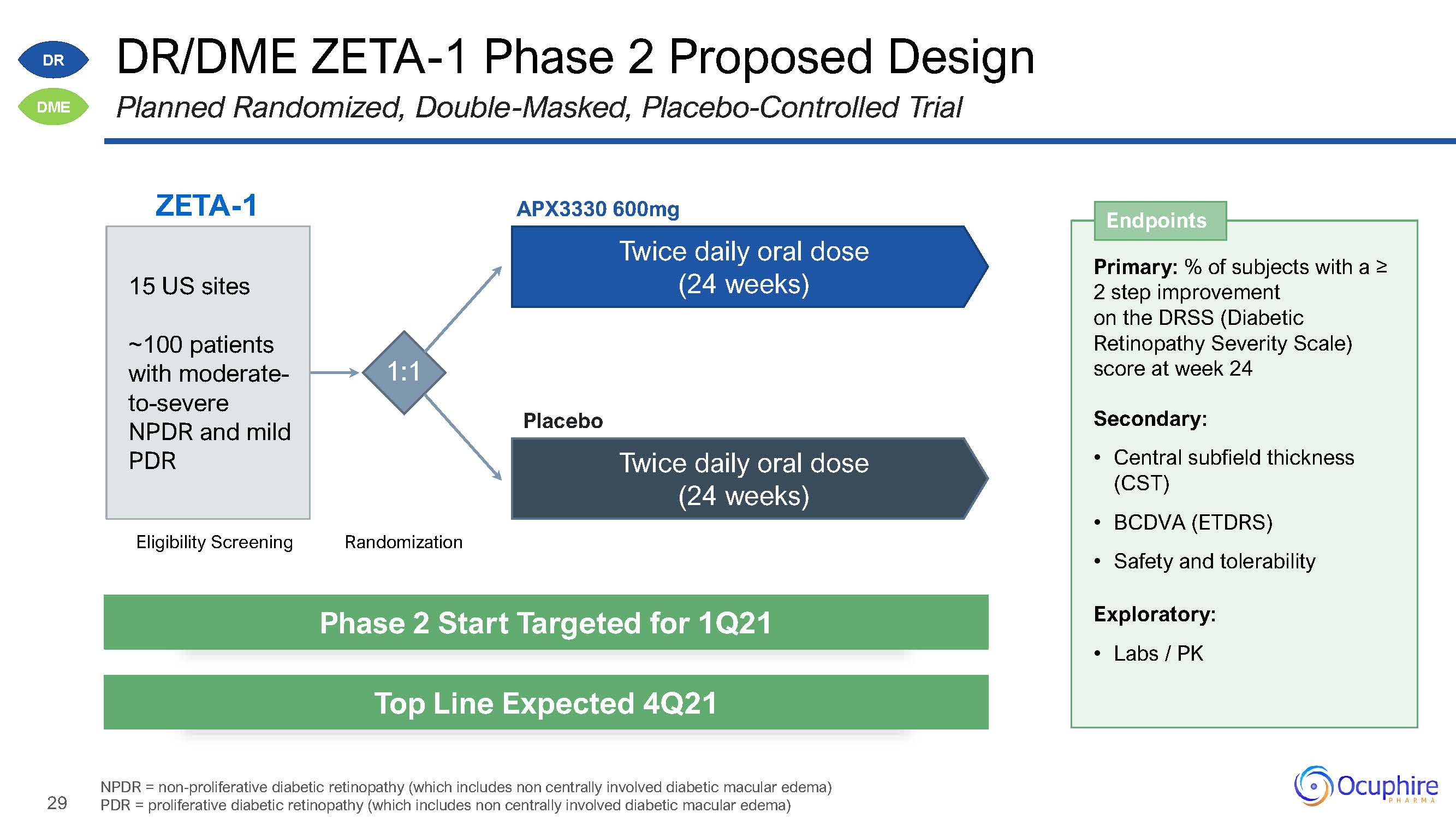
29 DR/DME ZETA-1 Phase 2 Proposed DesignPlanned Randomized, Double-Masked, Placebo-Controlled
Trial NPDR = non-proliferative diabetic retinopathy (which includes non centrally involved diabetic macular edema) PDR = proliferative diabetic retinopathy (which includes non centrally involved diabetic macular
edema) ZETA-1 Eligibility Screening Randomization 1:1 APX3330 600mgTwice daily oral dose(24 weeks) Twice daily oral dose (24 weeks) Placebo 15 US sites~100 patients with moderate- to-severe NPDR and mild
PDR DR DME Primary: % of subjects with a ≥2 step improvement on the DRSS (DiabeticRetinopathy Severity Scale) score at week 24Secondary:Central subfield thickness (CST)BCDVA (ETDRS)Safety and tolerabilityExploratory:Labs /
PK Endpoints Phase 2 Start Targeted for 1Q21 Top Line Expected 4Q21
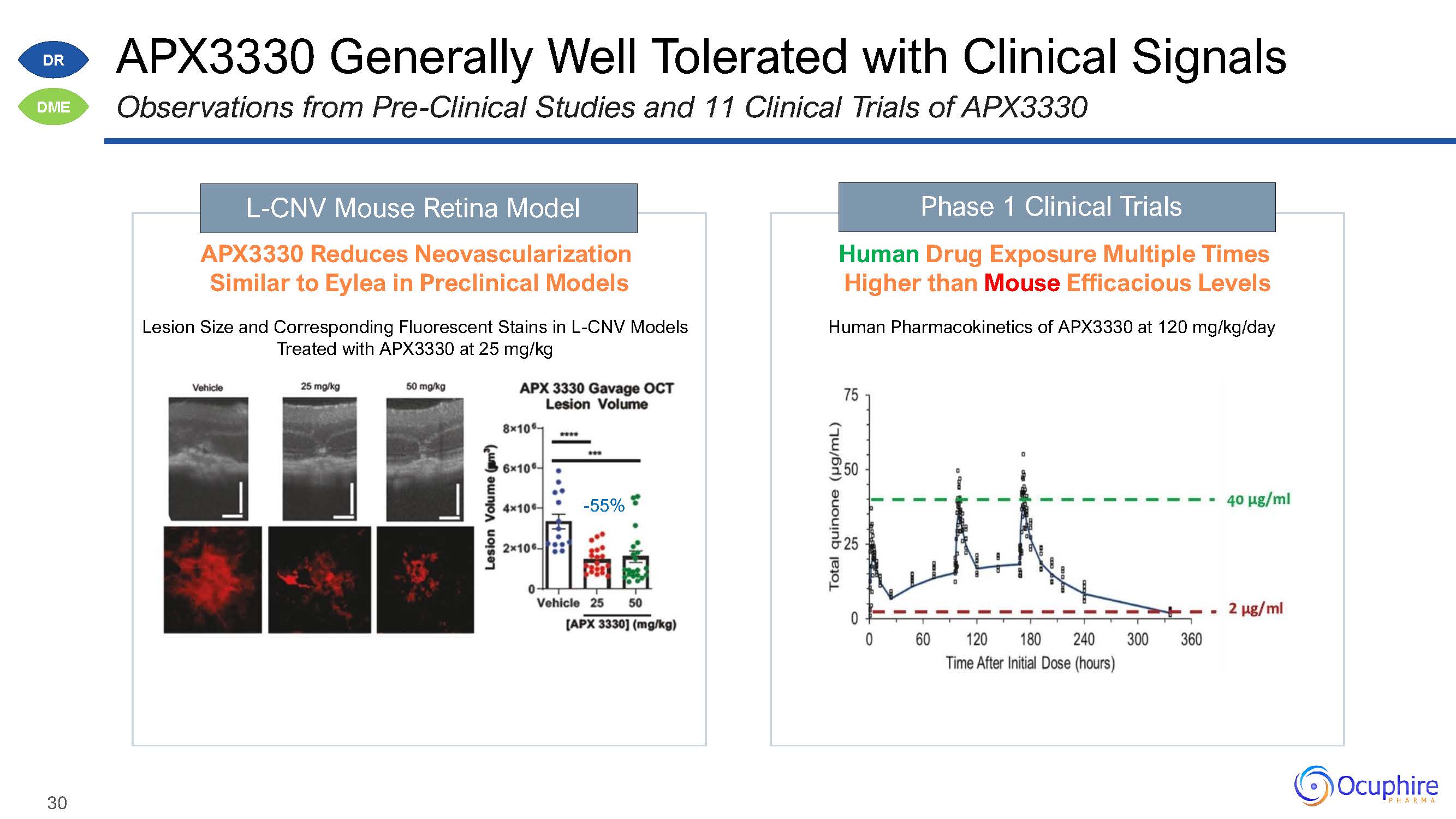
APX3330 Generally Well Tolerated with Clinical SignalsObservations from Pre-Clinical Studies and
11 Clinical Trials of APX3330 L-CNV Mouse Retina Model APX3330 Reduces Neovascularization Similar to Eylea in Preclinical ModelsLesion Size and Corresponding Fluorescent Stains in L-CNV Models Treated with APX3330 at 25
mg/kg-55% Phase 1 Clinical Trials Human Drug Exposure Multiple Times Higher than Mouse Efficacious LevelsHuman Pharmacokinetics of APX3330 at 120 mg/kg/day DR DME 30

Boards and Milestones 31

Richard Lindstrom, MD University of Minnesota Ed Holland, MDLoyola University
Chicago Jay Pepose, MD UCLA Jack Holladay, MD University of Texas Thomas Samuelson, MD University of Minnesota Paul Karpecki, OD Indiana University elCON MedicalEliot Lazar, MDGeorgetown University Gary Novak,
PhD UC Davis Marguerite McDonald, MD Columbia University David Boyer, MD Chicago Medical School Gerald Horn, MD University of IllinoisCo-Founder Ocularis/Nyxol Mark Kelley, PhD Indiana UniversityCo-Founder
Apexian/APX3330 Prestigious Ocular Medical Advisory BoardFortunate for the Insights of Leading KOLs & Drug Candidate Co-Founders Michael Allingham, MD, PhD University of North Carolina Richard Messmann, MD Wayne State
University CMO Apexian/APX3330 Peter Kaiser, MD Harvard Medical School Jeffrey Heier, MD Boston University 32

Ocuphire Board of DirectorsSeasoned Directors with Decades of Biotech Drug Development
and M&A/Financings Sean Ainsworth, MBALead Independent Director,Board Director James Manuso, PhD/MBABoard Director Alan R. Meyer, MBABoard Director MFR/IP Advisor Richard Rodgers,
MBABoard Director Susan Benton, MBABoard Director Cam Gallagher, MBAChair, Board Director Mina Sooch, MBA Vice-Chair, Board DirectorPresident & CEO 33
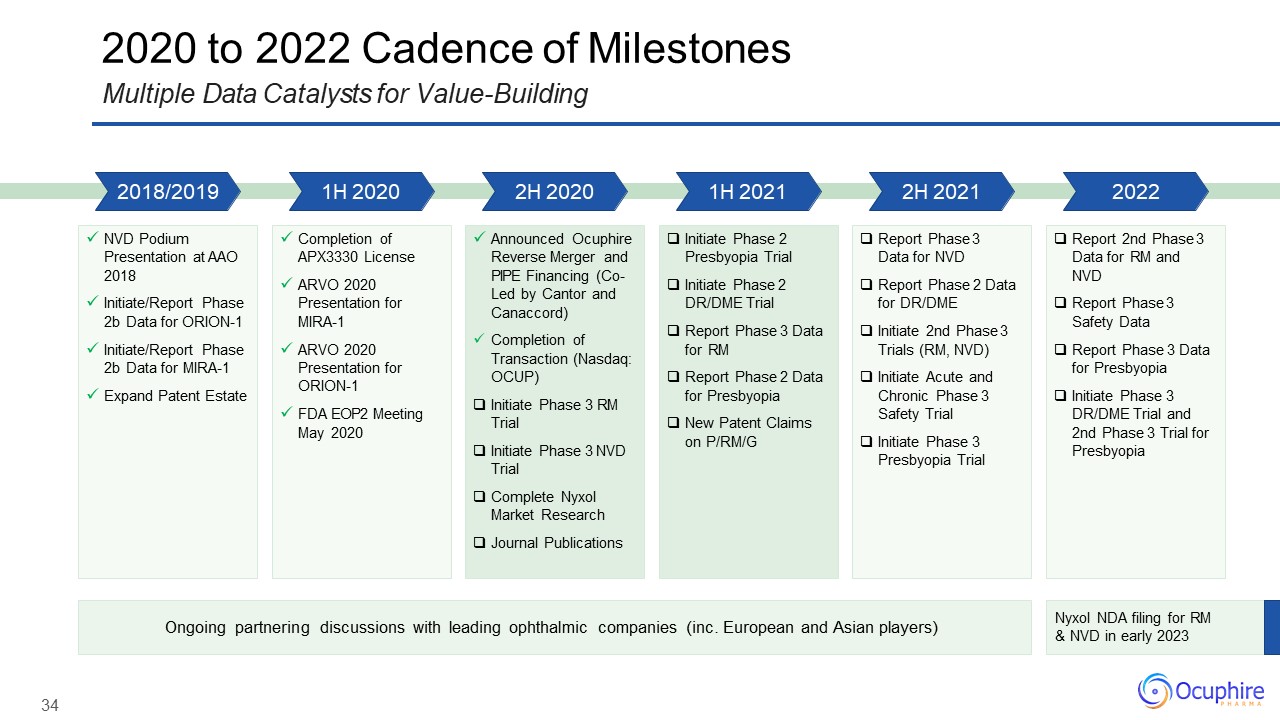
2020 to 2022 Cadence of MilestonesMultiple Data Catalysts for Value-Building Nyxol NDA
filing for RM & NVD in early 2023 Ongoing partnering discussions with leading ophthalmic companies (inc. European and Asian players) NVD Podium Presentation at AAO 2018Initiate/Report Phase 2b Data for ORION-1Initiate/Report Phase
2b Data for MIRA-1Expand Patent Estate Completion of APX3330 LicenseARVO 2020Presentation for MIRA-1ARVO 2020Presentation for ORION-1FDA EOP2 Meeting May 2020 Announced Ocuphire Reverse Merger and PIPE Financing (Co- Led by Cantor and
Canaccord)Completion of Transaction (Nasdaq: OCUP)Initiate Phase 3 RM TrialInitiate Phase 3 NVD TrialComplete Nyxol Market ResearchJournal Publications Initiate Phase 2 Presbyopia TrialInitiate Phase 2 DR/DME TrialReport Phase 3 Data for
RMReport Phase 2 Data for PresbyopiaNew Patent Claims on P/RM/G Report Phase 3 Data for NVDReport Phase 2 Data for DR/DMEInitiate 2nd Phase 3 Trials (RM, NVD)Initiate Acute and Chronic Phase 3 Safety TrialInitiate Phase 3 Presbyopia
Trial Report 2nd Phase 3 Data for RM and NVDReport Phase 3 Safety DataReport Phase 3 Data for PresbyopiaInitiate Phase 3 DR/DME Trial and 2nd Phase 3 Trial for Presbyopia 2018/2019 1H 2020 2H 2020 1H 2021 2H
2021 2022 34

Restore Vision & Clarity www.ocuphire.com ir@ocuphire.com
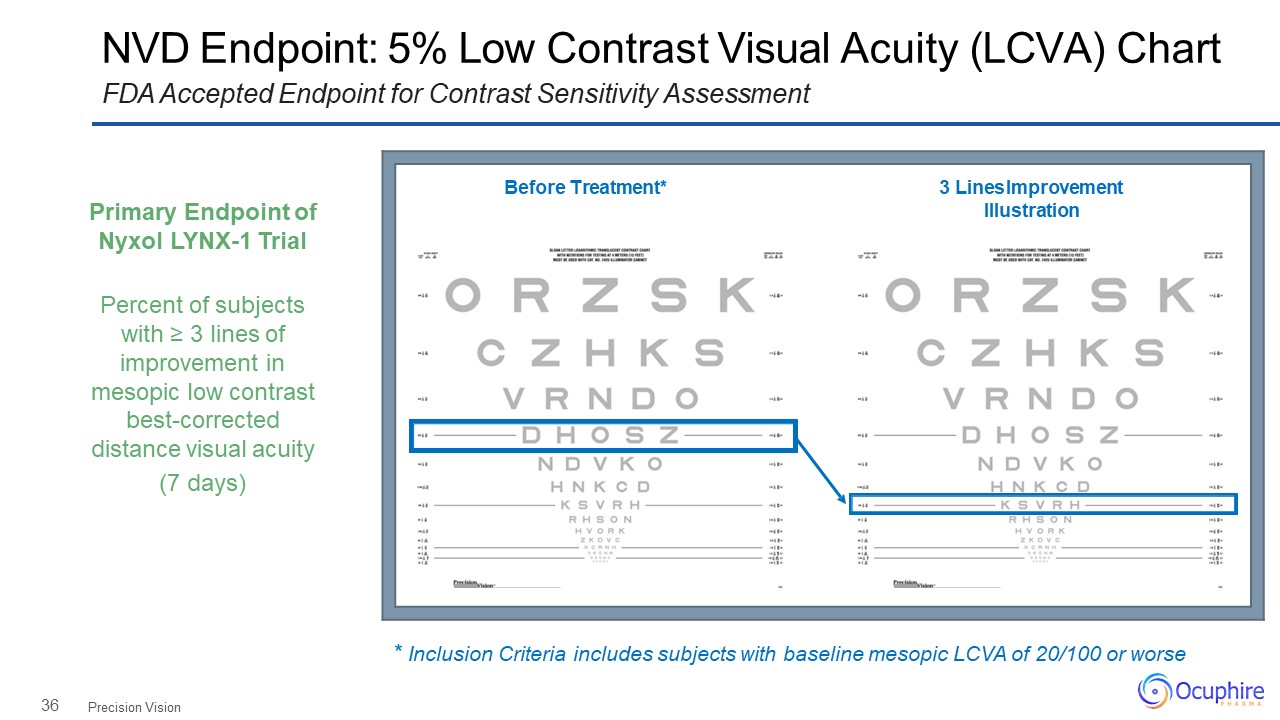
36 NVD Endpoint: 5% Low Contrast Visual Acuity (LCVA) ChartFDA Accepted Endpoint for Contrast
Sensitivity Assessment Precision Vision Before Treatment* 3 Lines Improvement Illustration Primary Endpoint of Nyxol LYNX-1 TrialPercent of subjects with ≥ 3 lines of improvement in mesopic low contrast
best-corrected distance visual acuity(7 days) * Inclusion Criteria includes subjects with baseline mesopic LCVA of 20/100 or worse
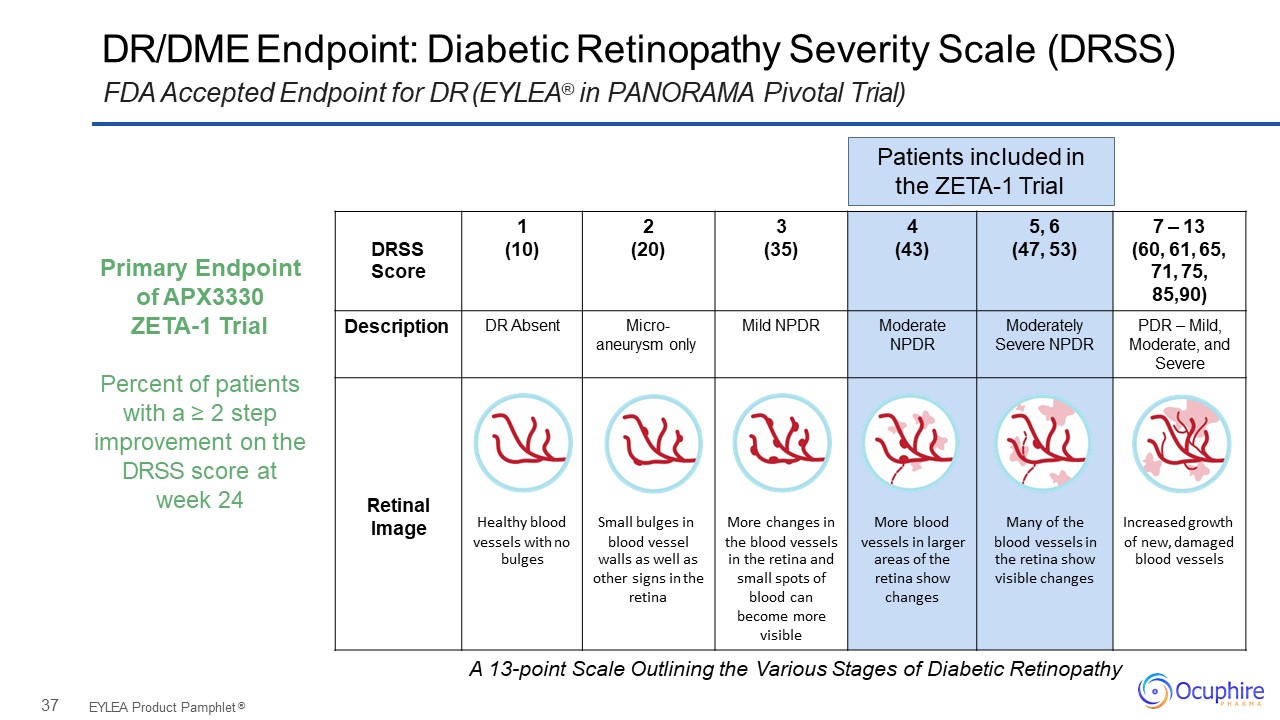
37 DR/DME Endpoint: Diabetic Retinopathy Severity Scale (DRSS)FDA Accepted Endpoint for DR
(EYLEA® in PANORAMA Pivotal Trial) EYLEA Product Pamphlet ® 1 2 3 4 5, 6 7 – 13 DRSS (10) (20) (35) (43) (47, 53) (60, 61, 65, Score 71, 75, 85,90) Description DR Absent Micro- aneurysm
only Mild NPDR Moderate NPDR Moderately Severe NPDR PDR – Mild, Moderate, and Severe Retinal Image Healthy blood vessels with no Small bulges in blood vessel More changes in the blood vessels More blood vessels in larger Many of
the blood vessels in Increased growth of new, damaged bulges walls as well as in the retina and areas of the the retina show blood vessels other signs in the small spots of retina show visible changes retina blood
can changes become more visible Patients included in the ZETA-1 Trial Primary Endpoint of APX3330ZETA-1 TrialPercent of patients with a ≥ 2 step improvement on the DRSS score at week 24 A
13-point Scale Outlining the Various Stages of Diabetic Retinopathy
www.ocuphire.comir@ocuphire.com