EXHIBIT 99.1
Published on June 30, 2021
Exhibit 99.1

VEGA-1 Phase 2 Topline Results Conference Call June 30, 2021

Disclosures and Forward Looking Statements This presentation contains “forward-looking statements”
within the meaning of the Private Securities Litigation Reform Act of 1995. Such statements include, but are not limited to, statements concerning Ocuphire Pharma, Inc.’s (“Ocuphire” or the “Company”) product candidates and future milestones,
including the potential for Nyxol to be a “best in class” presbyopia drop. These forward-looking statements are based upon the Company’s current expectations and involve assumptions that may never materialize or may prove to be incorrect.
Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, including, without limitation: (i) timing or ability for the company to
achieve its targeted milestones; (ii) the success and timing of regulatory submissions and pre-clinical and clinical trials; (iii) regulatory requirements or developments; (iv) changes to clinical trial designs and regulatory pathways; (v)
changes in capital resource requirements; (vi) risks related to the inability of the Company to obtain sufficient additional capital to continue to advance its product candidates and its preclinical programs; (vii) legislative, regulatory,
political and economic developments, and (viii) the effects of COVID-19 on clinical programs and business operations. The foregoing review of important factors that could cause actual events to differ from expectations should not be construed
as exhaustive and should be read in conjunction with statements that are included herein and elsewhere, including the risk factors detailed in documents that have been and may be filed by the Company from time to time with the SEC. All
forward-looking statements contained in this presentation speak only as of the date on which they were made. The Company undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date
on which they were made. The Company makes no representation or warranty, express or implied, as to the accuracy or completeness of the information contained in or incorporated by reference into this presentation. Nothing contained in or
incorporated by reference into this presentation is, or shall be relied upon as, a promise or representation by the Company as to the past or future. The Company assumes no responsibility for the accuracy or completeness of any such
information. This presentation may not be reproduced or provided to any other person (other than your advisor) without our prior written consent. By accepting delivery of this presentation, you agree to the foregoing and agree to return this
presentation and any documents related thereto and any copies thereof to us or to destroy the same if you do not make an investment in any securities. The information contain within this presentation shall not, except as hereinafter provided,
without the prior written consent of the Company, be disclosed by you or your representatives in any manner whatsoever, in whole or in part, and shall not be used by you or your representatives other than for the purpose of evaluating the
transaction described herein. By accepting delivery of this presentation you further acknowledge and agree aware of the restrictions imposed by the United States securities laws on the purchase or sale of securities by any person who has
received material, nonpublic information from the issuer of the securities or any affiliate thereof and on the communication of such information to any other person when it is reasonably foreseeable that such other person is likely to purchase
or sell such securities in reliance on such information for so long as the information remains material and non-public. This presentation also contains estimates and other statistical data made by independent parties and by us relating to
market shares and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. The trademarks included herein are the property of the owners
thereof and are used for reference purposes only. Such use should not be construed as an endorsement of such products. November 6, 2020
Agenda and Participants Topline VEGA-1 Phase 2 Clinical Trial Results for Nyxol and Low-Dose Pilocarpine
in PresbyopiaPresbyopia Market OpportunityFuture MilestonesQ&AParticipantsMina Sooch, MBA, President and CEOMitch Brigell, PhD, Head of Clinical DevelopmentJay Pepose, MD, Medical Advisory Board & Corporate Board MemberSusan Benton,
Corporate Board MemberCharlie Hoffmann, MBA, VP of Corporate Development and OperationsAmy Rabourn, MAcc, VP of Finance Phase 2 Trial Topline Readout As Planned In 2Q21
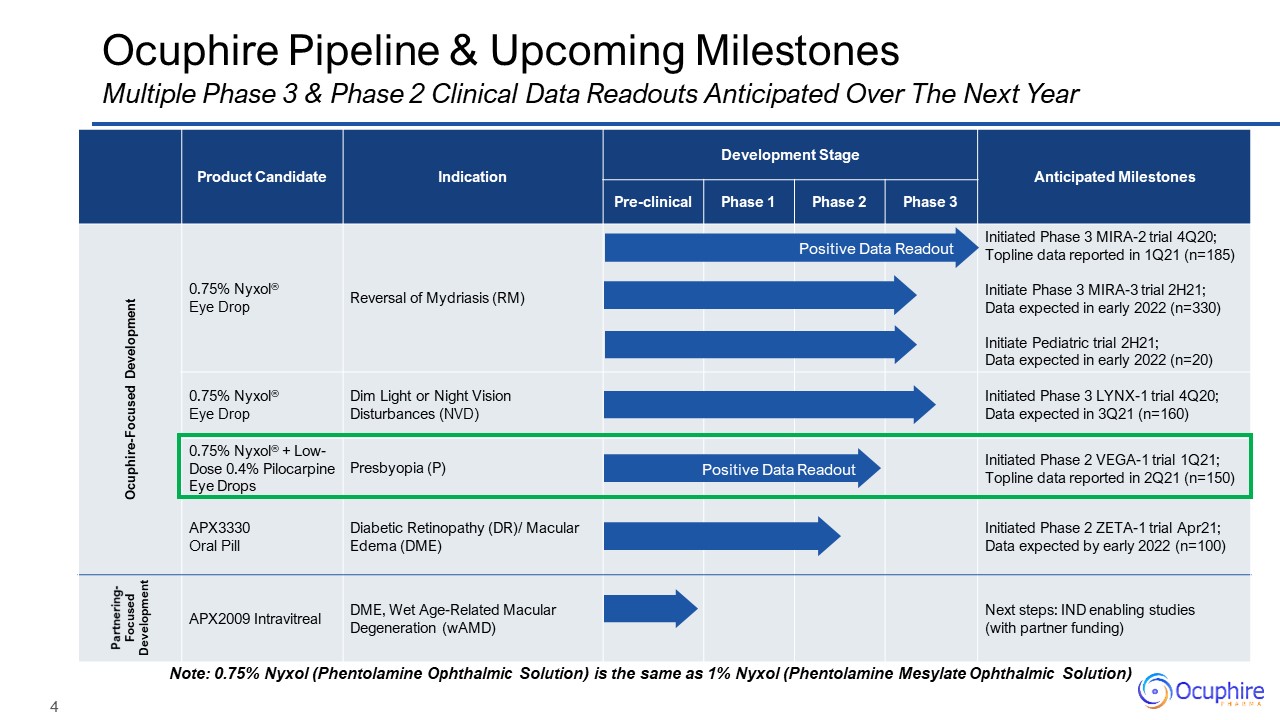
Product Candidate Indication Development Stage Anticipated
Milestones Pre-clinical Phase 1 Phase 2 Phase 3 Ocuphire-Focused Development 0.75% Nyxol®Eye Drop Reversal of Mydriasis (RM) Initiated Phase 3 MIRA-2 trial 4Q20; Topline data reported in 1Q21 (n=185)Initiate Phase 3
MIRA-3 trial 2H21; Data expected in early 2022 (n=330) Initiate Pediatric trial 2H21;Data expected in early 2022 (n=20) 0.75% Nyxol®Eye Drop Dim Light or Night Vision Disturbances (NVD) Initiated Phase 3 LYNX-1 trial 4Q20;Data
expected in 3Q21 (n=160) 0.75% Nyxol® + Low-Dose 0.4% Pilocarpine Eye Drops Presbyopia (P) Initiated Phase 2 VEGA-1 trial 1Q21;Topline data reported in 2Q21 (n=150) APX3330 Oral Pill Diabetic Retinopathy (DR)/ Macular Edema
(DME) Initiated Phase 2 ZETA-1 trial Apr21;Data expected by early 2022 (n=100) Partnering-Focused Development APX2009 Intravitreal DME, Wet Age-Related Macular Degeneration (wAMD) Next steps: IND enabling studies (with
partner funding) Ocuphire Pipeline & Upcoming Milestones Multiple Phase 3 & Phase 2 Clinical Data Readouts Anticipated Over The Next Year Note: 0.75% Nyxol (Phentolamine Ophthalmic Solution) is the same as 1% Nyxol (Phentolamine
Mesylate Ophthalmic Solution) Positive Data Readout Positive Data Readout
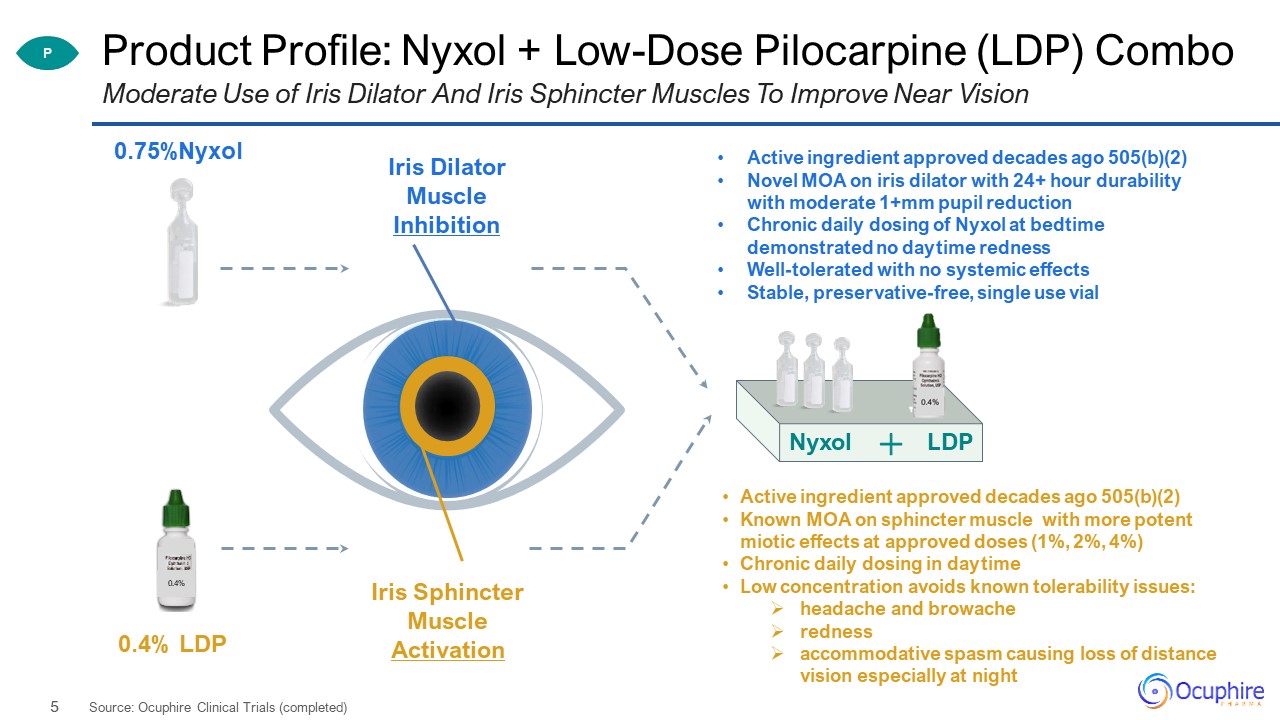
Product Profile: Nyxol + Low-Dose Pilocarpine (LDP) Combo Moderate Use of Iris Dilator And Iris
Sphincter Muscles To Improve Near Vision 0.4% 0.75%Nyxol 0.4% LDP Nyxol LDP P Iris Dilator Muscle Inhibition Iris Sphincter Muscle Activation Active ingredient approved decades ago 505(b)(2)Novel MOA on iris
dilator with 24+ hour durability with moderate 1+mm pupil reductionChronic daily dosing of Nyxol at bedtime demonstrated no daytime rednessWell-tolerated with no systemic effectsStable, preservative-free, single use vial Active ingredient
approved decades ago 505(b)(2)Known MOA on sphincter muscle with more potent miotic effects at approved doses (1%, 2%, 4%)Chronic daily dosing in daytimeLow concentration avoids known tolerability issues:headache and
browacherednessaccommodative spasm causing loss of distance vision especially at night Source: Ocuphire Clinical Trials (completed)
Potential ‘Best in Class’ Presbyopia DropTopline Results From Vega-1 Were Positive… Nyxol + LDP
Presbyopia Treatment is Differentiated: Statistically significant efficacy dataFavorable safety profileComfort and tolerabilityFast onsetLong durationMaintain good distance visual acuity (night/day)Novel tunable pupil modulation P
Nyxol® Phentolamine Mesylate NVD P Presbyopia RM Night Vision Disturbances Reversal of
Mydriasis 0.4% +

Topline VEGA-1 Phase 2 Results Randomized, Multi-Center, Double-Masked, Placebo-Controlled Study of the
Safety and Efficacy of Nyxol (0.75% Phentolamine Ophthalmic Solution) + 0.4% Low Dose Pilocarpine (LDP) for the Treatment of Presbyopia Clinical trial NCT#04675151
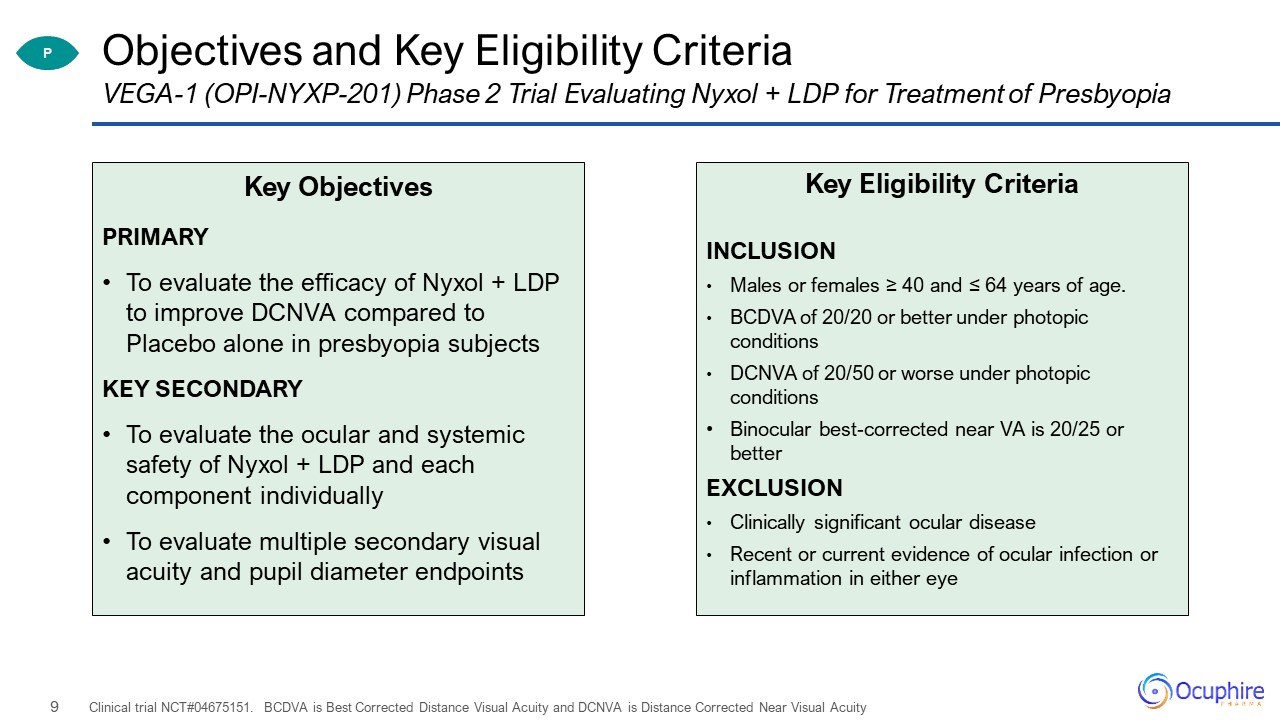
Objectives and Key Eligibility Criteria Key ObjectivesPRIMARYTo evaluate the efficacy of Nyxol + LDP to
improve DCNVA compared to Placebo alone in presbyopia subjectsKEY SECONDARYTo evaluate the ocular and systemic safety of Nyxol + LDP and each component individuallyTo evaluate multiple secondary visual acuity and pupil diameter
endpoints Clinical trial NCT#04675151. BCDVA is Best Corrected Distance Visual Acuity and DCNVA is Distance Corrected Near Visual Acuity VEGA-1 (OPI-NYXP-201) Phase 2 Trial Evaluating Nyxol + LDP for Treatment of Presbyopia Key Eligibility
CriteriaINCLUSIONMales or females ≥ 40 and ≤ 64 years of age.BCDVA of 20/20 or better under photopic conditionsDCNVA of 20/50 or worse under photopic conditionsBinocular best-corrected near VA is 20/25 or better EXCLUSIONClinically significant
ocular diseaseRecent or current evidence of ocular infection or inflammation in either eye P
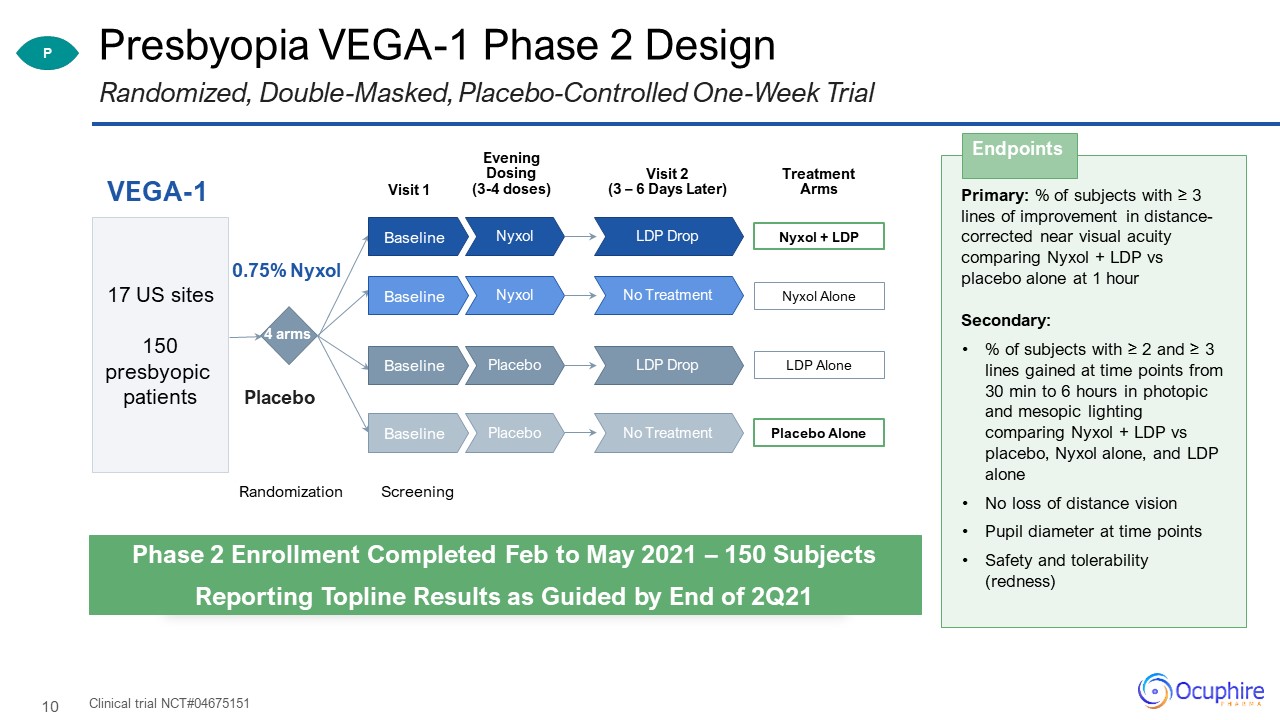
Presbyopia VEGA-1 Phase 2 Design Clinical trial NCT#04675151 Randomized, Double-Masked,
Placebo-Controlled One-Week Trial Primary: % of subjects with ≥ 3 lines of improvement in distance-corrected near visual acuity comparing Nyxol + LDP vs placebo alone at 1 hourSecondary:% of subjects with ≥ 2 and ≥ 3 lines gained at time
points from 30 min to 6 hours in photopic and mesopic lighting comparing Nyxol + LDP vs placebo, Nyxol alone, and LDP aloneNo loss of distance vision Pupil diameter at time pointsSafety and tolerability (redness) Endpoints P Phase 2
Enrollment Completed Feb to May 2021 – 150 SubjectsReporting Topline Results as Guided by End of 2Q21 Visit 1 VEGA-1 Randomization 4 arms 0.75% Nyxol Placebo 17 US sites150 presbyopic patients Visit 2 (3 – 6 Days
Later) Screening Treatment Arms Nyxol + LDP LDP Drop Nyxol Baseline Nyxol Alone No Treatment Nyxol Baseline LDP Alone LDP Drop Placebo Baseline Placebo Alone No Treatment Placebo Baseline Evening Dosing(3-4 doses)
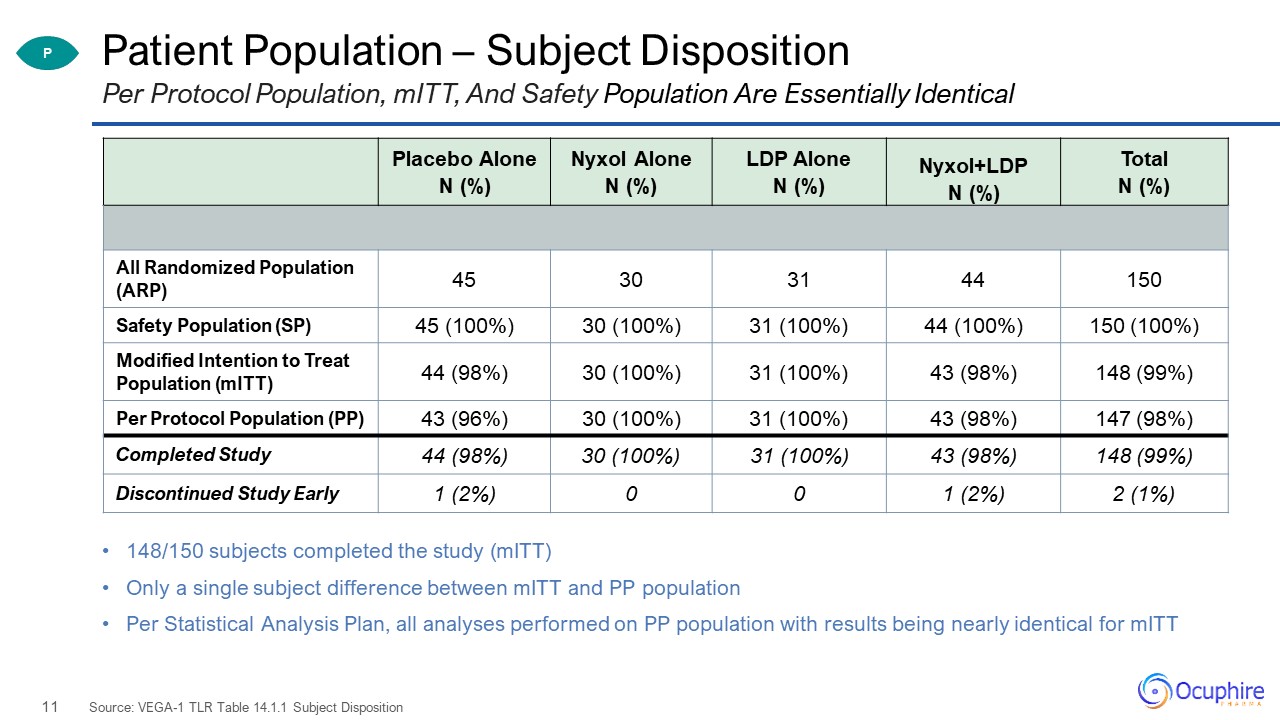
Patient Population – Subject Disposition 148/150 subjects completed the study (mITT)Only a single
subject difference between mITT and PP populationPer Statistical Analysis Plan, all analyses performed on PP population with results being nearly identical for mITT Source: VEGA-1 TLR Table 14.1.1 Subject Disposition Per Protocol Population,
mITT, And Safety Population Are Essentially Identical Placebo Alone N (%) Nyxol Alone N (%) LDP Alone N (%) Nyxol+LDP N (%) Total N (%) All Randomized Population (ARP) 45 30 31 44 150 Safety Population (SP) 45
(100%) 30 (100%) 31 (100%) 44 (100%) 150 (100%) Modified Intention to Treat Population (mITT) 44 (98%) 30 (100%) 31 (100%) 43 (98%) 148 (99%) Per Protocol Population (PP) 43 (96%) 30 (100%) 31 (100%) 43 (98%) 147
(98%) Completed Study 44 (98%) 30 (100%) 31 (100%) 43 (98%) 148 (99%) Discontinued Study Early 1 (2%) 0 0 1 (2%) 2 (1%) P
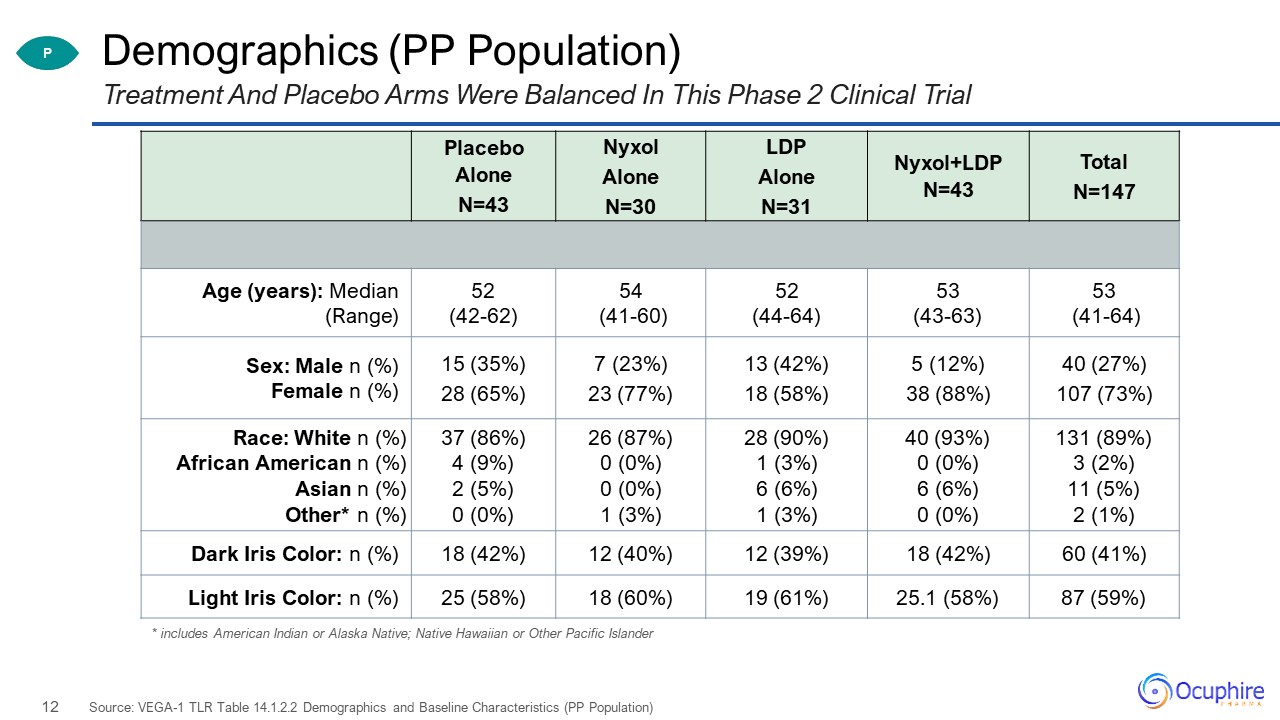
Demographics (PP Population) Treatment And Placebo Arms Were Balanced In This Phase 2 Clinical
Trial Source: VEGA-1 TLR Table 14.1.2.2 Demographics and Baseline Characteristics (PP Population) P Placebo Alone N=43 Nyxol Alone N=30 LDP Alone N=31 Nyxol+LDP N=43 Total N=147 Age (years): Median (Range) 52 (42-62) 54
(41-60) 52 (44-64) 53 (43-63) 53 (41-64) Sex: Male n (%) Female n (%) 15 (35%)28 (65%) 7 (23%)23 (77%) 13 (42%)18 (58%) 5 (12%)38 (88%) 40 (27%)107 (73%) Race: White n (%)African American n (%)Asian n (%)Other* n (%) 37 (86%)4 (9%)2
(5%)0 (0%) 26 (87%)0 (0%)0 (0%)1 (3%) 28 (90%)1 (3%)6 (6%)1 (3%) 40 (93%)0 (0%)6 (6%)0 (0%) 131 (89%)3 (2%)11 (5%)2 (1%) Dark Iris Color: n (%) 18 (42%) 12 (40%) 12 (39%) 18 (42%) 60 (41%) Light Iris Color: n (%) 25 (58%) 18
(60%) 19 (61%) 25.1 (58%) 87 (59%) * includes American Indian or Alaska Native; Native Hawaiian or Other Pacific Islander
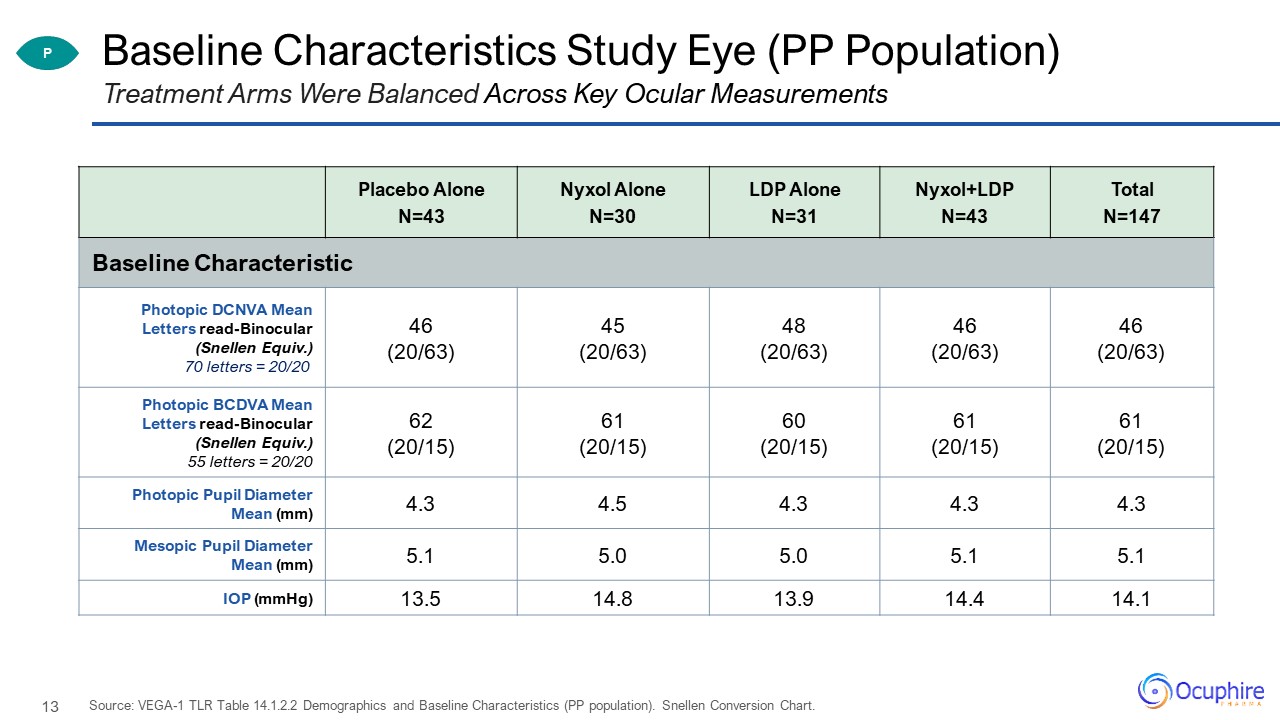
Baseline Characteristics Study Eye (PP Population) Placebo Alone N=43 Nyxol AloneN=30 LDP Alone
N=31 Nyxol+LDPN=43 TotalN=147 Baseline Characteristic Photopic DCNVA Mean Letters read-Binocular (Snellen Equiv.)70 letters = 20/20 46 (20/63) 45 (20/63) 48(20/63) 46 (20/63) 46 (20/63) Photopic BCDVA Mean Letters
read-Binocular (Snellen Equiv.)55 letters = 20/20 62 (20/15) 61 (20/15) 60 (20/15) 61 (20/15) 61(20/15) Photopic Pupil Diameter Mean (mm) 4.3 4.5 4.3 4.3 4.3 Mesopic Pupil Diameter Mean (mm) 5.1 5.0 5.0 5.1 5.1 IOP
(mmHg) 13.5 14.8 13.9 14.4 14.1 Treatment Arms Were Balanced Across Key Ocular Measurements Source: VEGA-1 TLR Table 14.1.2.2 Demographics and Baseline Characteristics (PP population). Snellen Conversion Chart. P
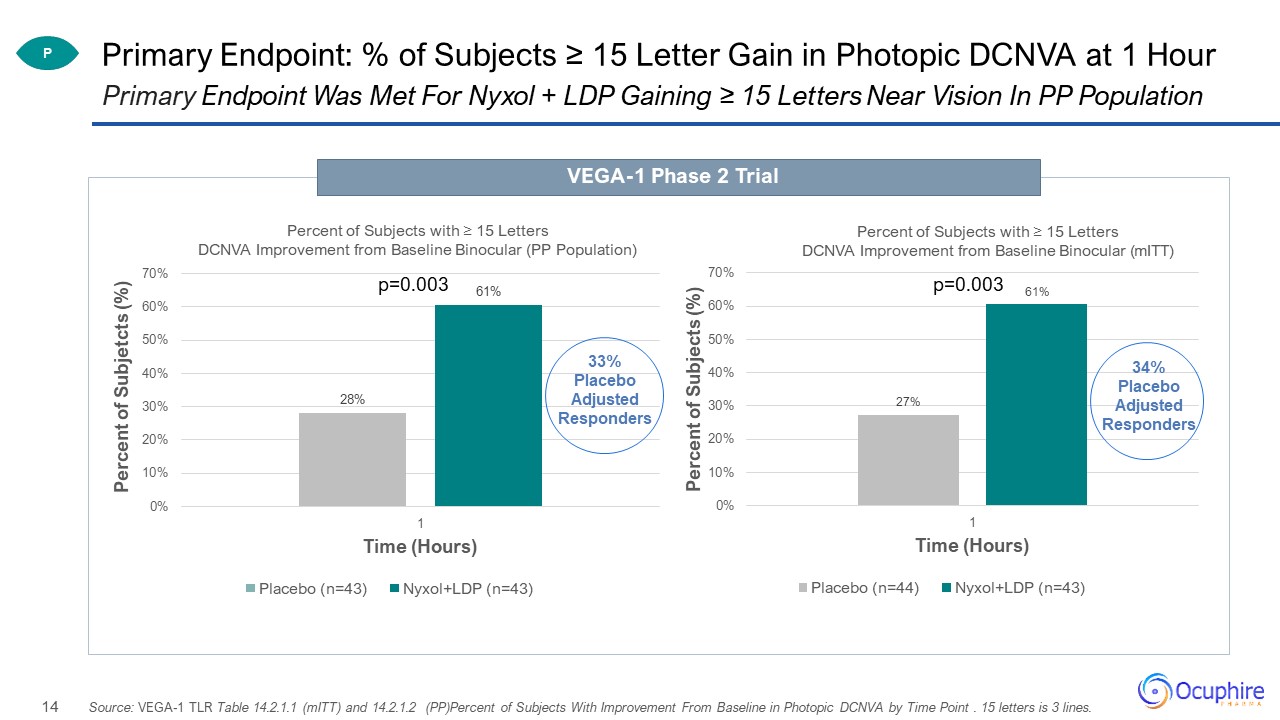
Primary Endpoint: % of Subjects ≥ 15 Letter Gain in Photopic DCNVA at 1 Hour Primary Endpoint Was Met
For Nyxol + LDP Gaining ≥ 15 Letters Near Vision In PP Population VEGA-1 Phase 2 Trial P Source: VEGA-1 TLR Table 14.2.1.1 (mITT) and 14.2.1.2 (PP)Percent of Subjects With Improvement From Baseline in Photopic DCNVA by Time Point . 15
letters is 3 lines. p=0.003 34% Placebo Adjusted Responders
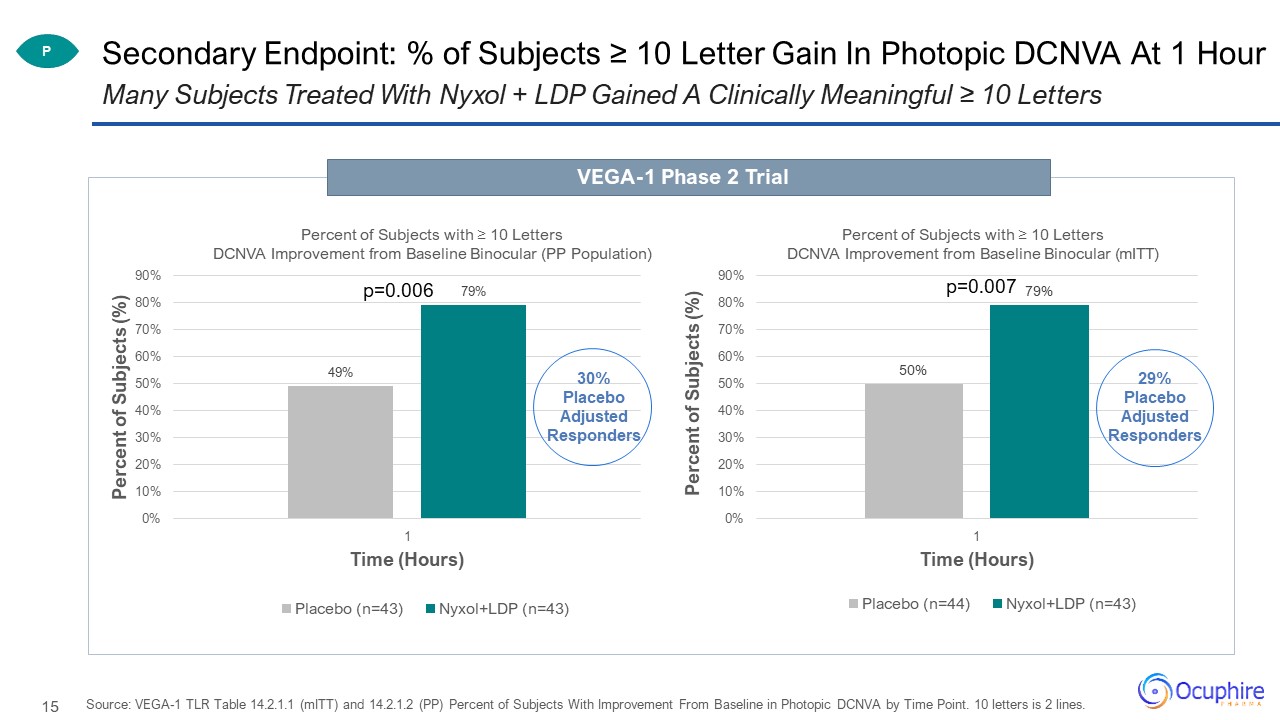
Secondary Endpoint: % of Subjects ≥ 10 Letter Gain In Photopic DCNVA At 1 Hour Source: VEGA-1 TLR Table
14.2.1.1 (mITT) and 14.2.1.2 (PP) Percent of Subjects With Improvement From Baseline in Photopic DCNVA by Time Point. 10 letters is 2 lines. Many Subjects Treated With Nyxol + LDP Gained A Clinically Meaningful ≥ 10 Letters VEGA-1 Phase 2
Trial P p=0.006 p=0.007 30%Placebo Adjusted Responders 29%Placebo Adjusted Responders
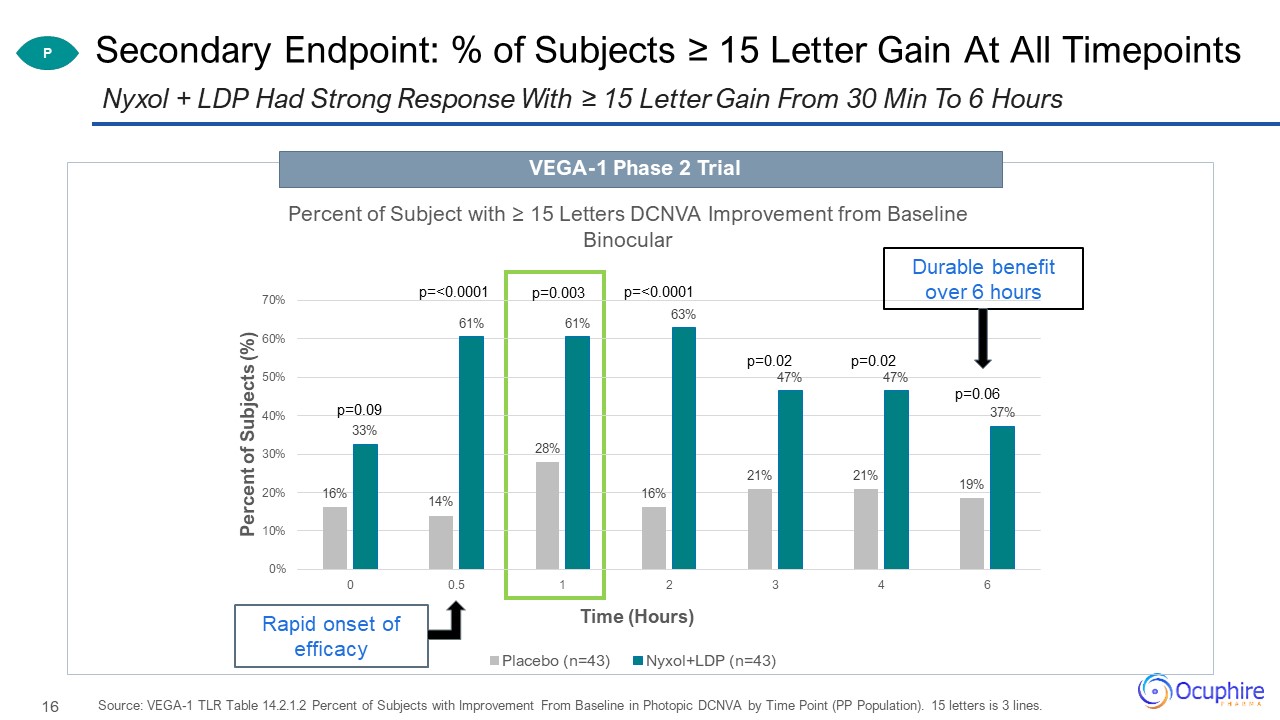
Secondary Endpoint: % of Subjects ≥ 15 Letter Gain At All Timepoints Source: VEGA-1 TLR Table 14.2.1.2
Percent of Subjects with Improvement From Baseline in Photopic DCNVA by Time Point (PP Population). 15 letters is 3 lines. Nyxol + LDP Had Strong Response With ≥ 15 Letter Gain From 30 Min To 6 Hours P Durable benefit over 6 hours Rapid
onset of efficacy VEGA-1 Phase 2 Trial
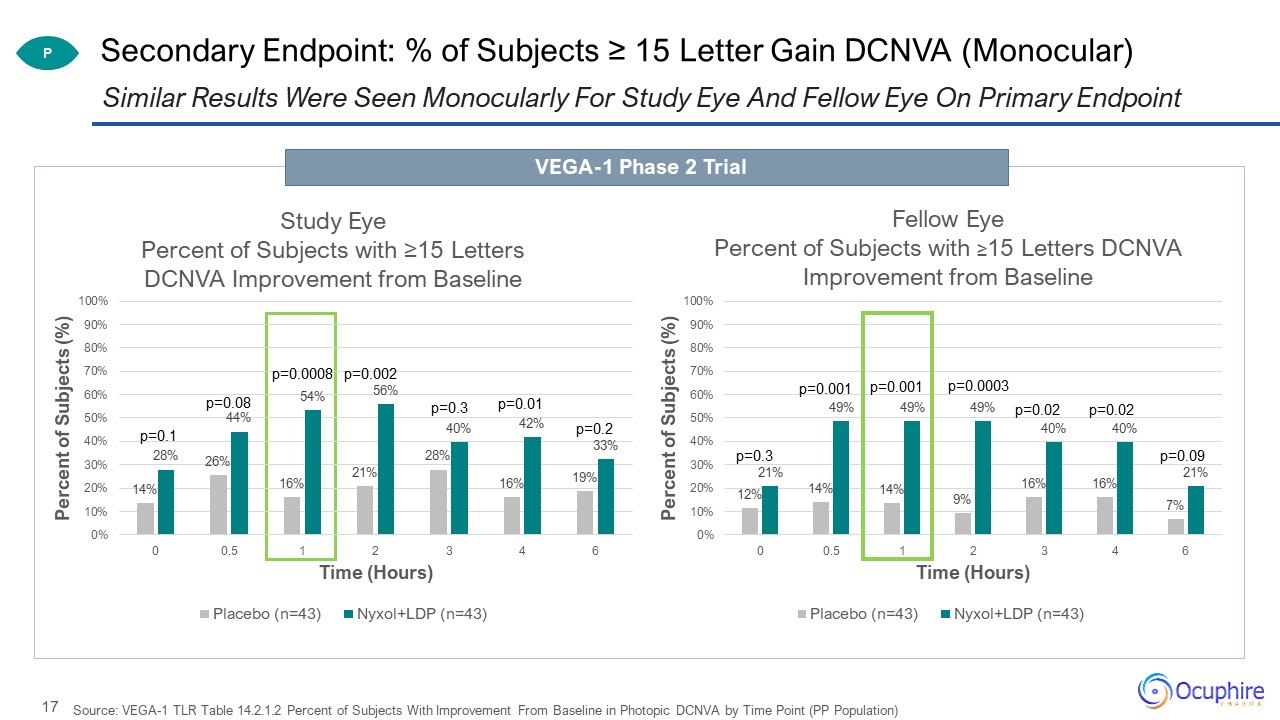
Source: VEGA-1 TLR Table 14.2.1.2 Percent of Subjects With Improvement From Baseline in Photopic DCNVA by
Time Point (PP Population) Similar Results Were Seen Monocularly For Study Eye And Fellow Eye On Primary Endpoint Secondary Endpoint: % of Subjects ≥ 15 Letter Gain DCNVA (Monocular) P VEGA-1 Phase 2 Trial
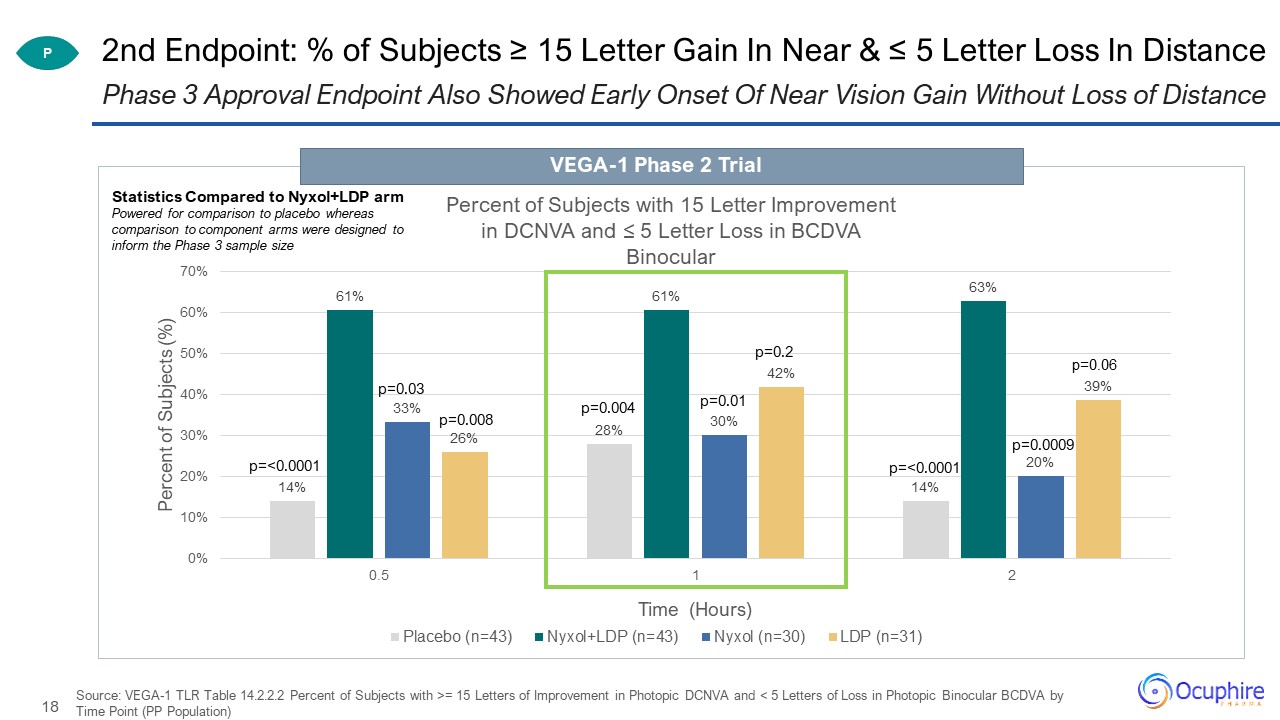
2nd Endpoint: % of Subjects ≥ 15 Letter Gain In Near & ≤ 5 Letter Loss In Distance Source: VEGA-1
TLR Table 14.2.2.2 Percent of Subjects with >= 15 Letters of Improvement in Photopic DCNVA and < 5 Letters of Loss in Photopic Binocular BCDVA by Time Point (PP Population) Phase 3 Approval Endpoint Also Showed Early Onset Of Near Vision
Gain Without Loss of Distance P p=0.0009 Statistics Compared to Nyxol+LDP armPowered for comparison to placebo whereas comparison to component arms were designed to inform the Phase 3 sample size VEGA-1 Phase 2 Trial
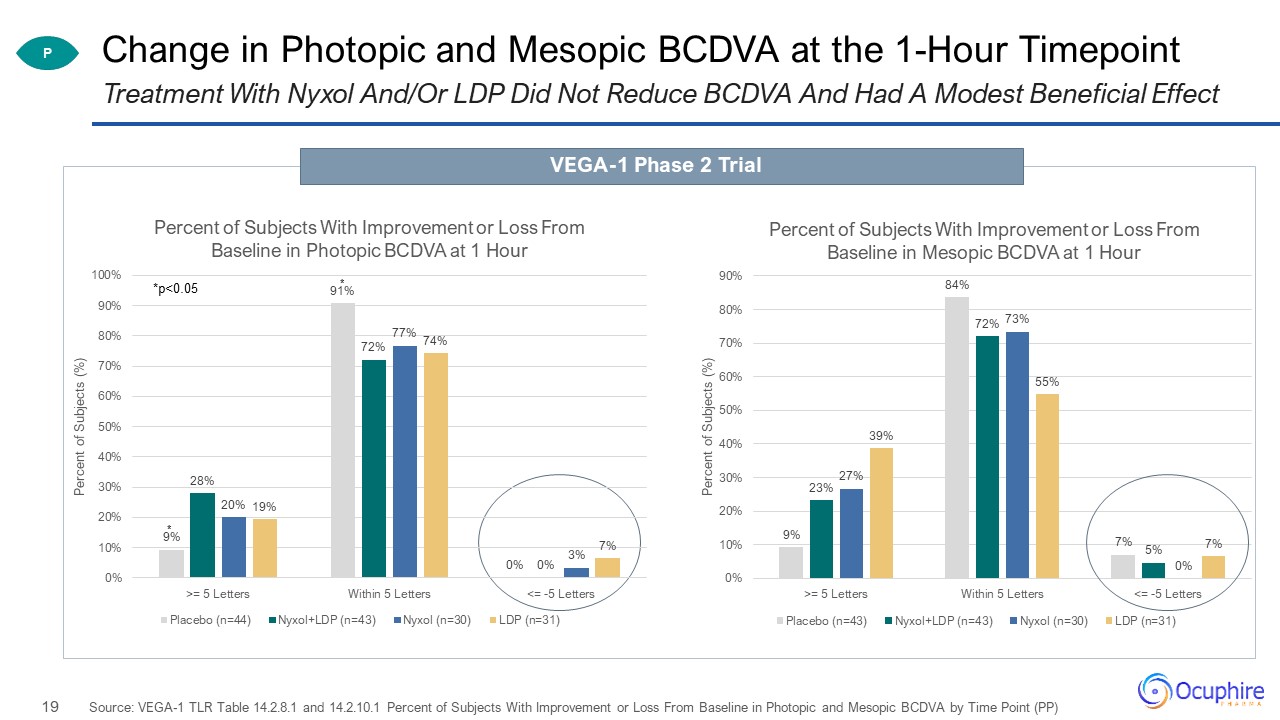
Change in Photopic and Mesopic BCDVA at the 1-Hour Timepoint Source: VEGA-1 TLR Table 14.2.8.1 and
14.2.10.1 Percent of Subjects With Improvement or Loss From Baseline in Photopic and Mesopic BCDVA by Time Point (PP) Treatment With Nyxol And/Or LDP Did Not Reduce BCDVA And Had A Modest Beneficial Effect P VEGA-1 Phase 2 Trial
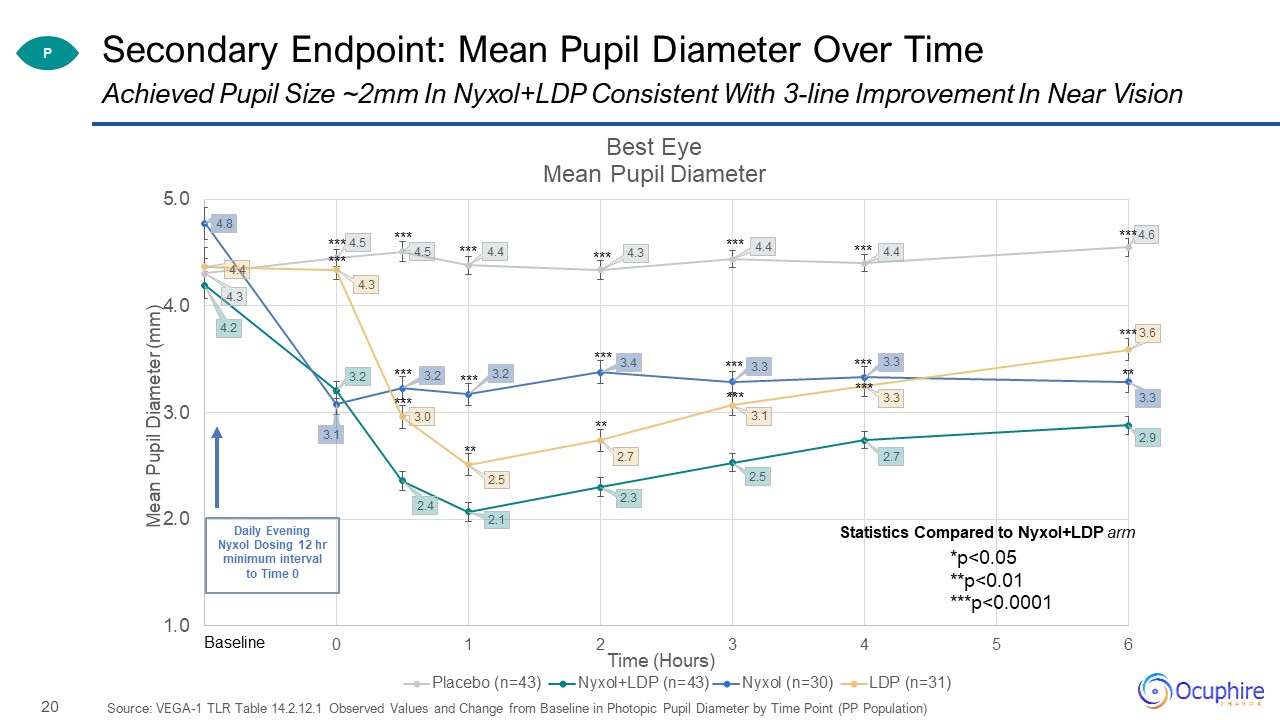
Secondary Endpoint: Mean Pupil Diameter Over Time Source: VEGA-1 TLR Table 14.2.12.1 Observed Values and
Change from Baseline in Photopic Pupil Diameter by Time Point (PP Population) Achieved Pupil Size ~2mm In Nyxol+LDP Consistent With 3-line Improvement In Near Vision P Statistics Compared to Nyxol+LDP arm
*p<0.05**p<0.01***p<0.0001
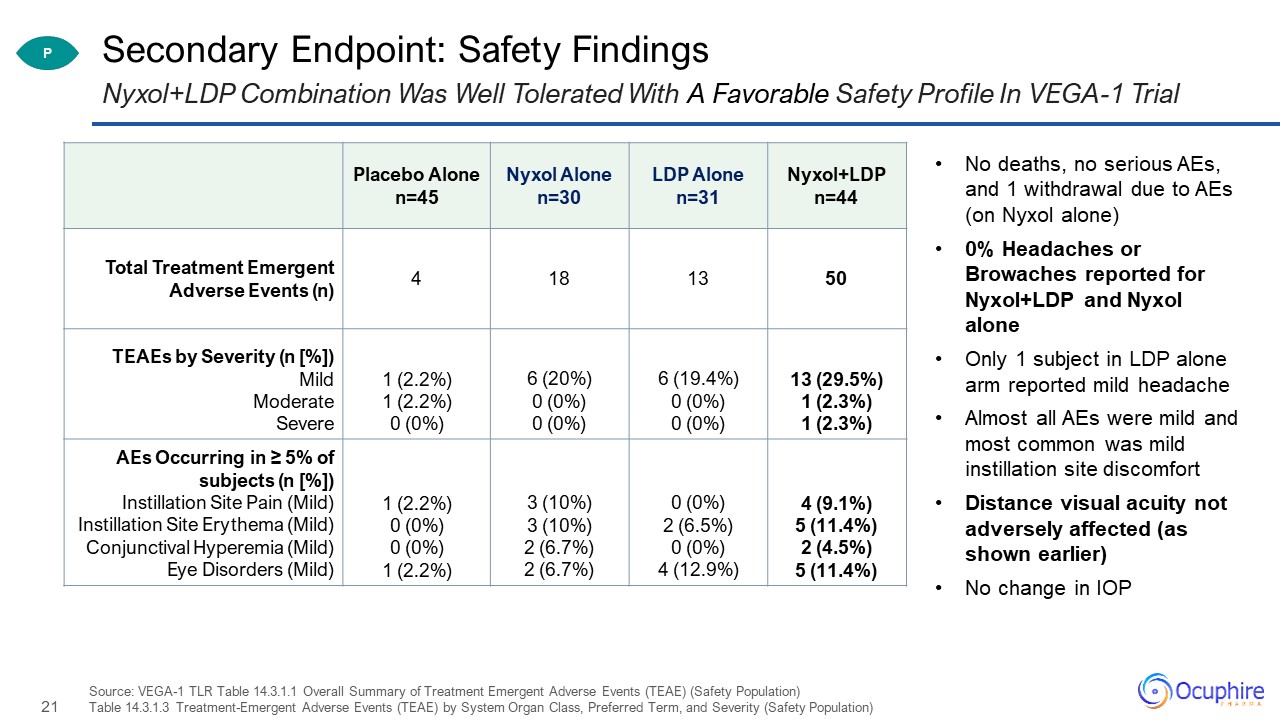
Secondary Endpoint: Safety Findings Source: VEGA-1 TLR Table 14.3.1.1 Overall Summary of Treatment
Emergent Adverse Events (TEAE) (Safety Population)Table 14.3.1.3 Treatment-Emergent Adverse Events (TEAE) by System Organ Class, Preferred Term, and Severity (Safety Population) Nyxol+LDP Combination Was Well Tolerated With A Favorable Safety
Profile In VEGA-1 Trial Placebo Alonen=45 Nyxol Alonen=30 LDP Alonen=31 Nyxol+LDPn=44 Total Treatment Emergent Adverse Events (n) 4 18 13 50 TEAEs by Severity (n [%])MildModerateSevere 1 (2.2%)1 (2.2%)0 (0%) 6 (20%)0
(0%)0 (0%) 6 (19.4%)0 (0%)0 (0%) 13 (29.5%)1 (2.3%)1 (2.3%) AEs Occurring in ≥ 5% of subjects (n [%])Instillation Site Pain (Mild)Instillation Site Erythema (Mild)Conjunctival Hyperemia (Mild)Eye Disorders (Mild) 1 (2.2%)0
(0%)0 (0%)1 (2.2%) 3 (10%)3 (10%)2 (6.7%)2 (6.7%) 0 (0%)2 (6.5%)0 (0%)4 (12.9%) 4 (9.1%)5 (11.4%)2 (4.5%)5 (11.4%) No deaths, no serious AEs, and 1 withdrawal due to AEs (on Nyxol alone)0% Headaches or Browaches reported for
Nyxol+LDP and Nyxol aloneOnly 1 subject in LDP alone arm reported mild headacheAlmost all AEs were mild and most common was mild instillation site discomfortDistance visual acuity not adversely affected (as shown earlier)No change in IOP P
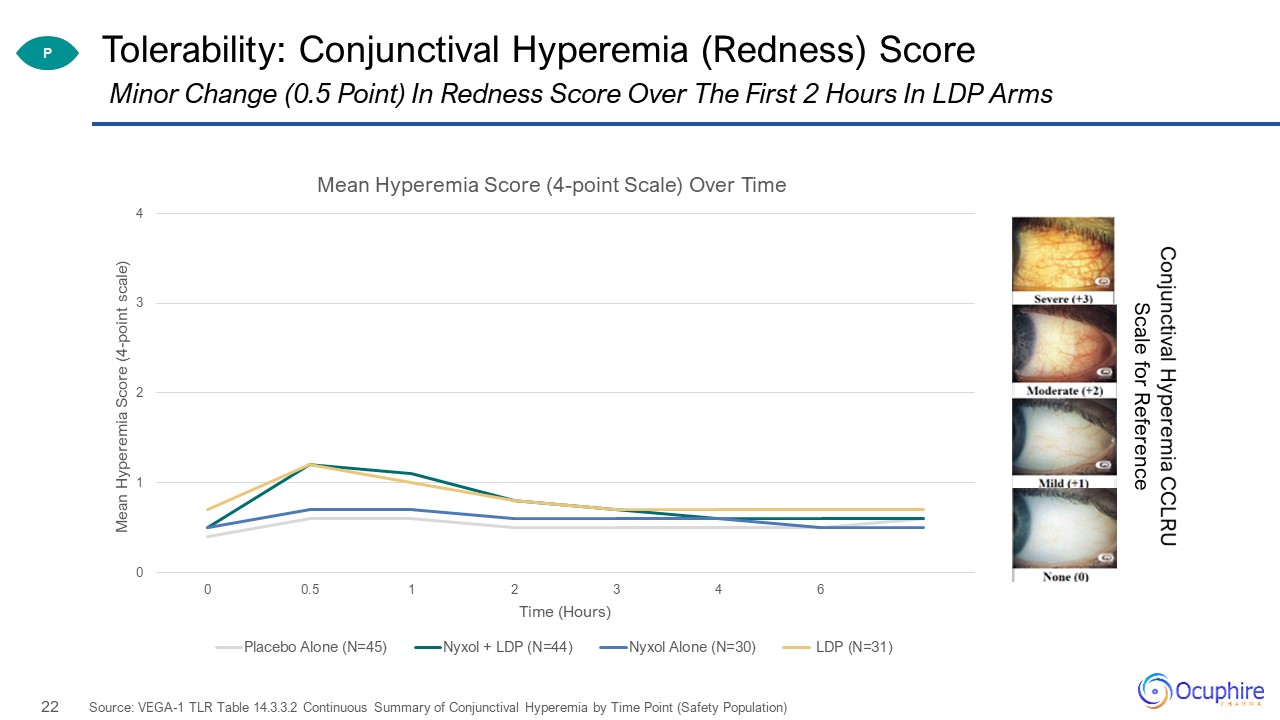
Tolerability: Conjunctival Hyperemia (Redness) Score Source: VEGA-1 TLR Table 14.3.3.2 Continuous
Summary of Conjunctival Hyperemia by Time Point (Safety Population) Minor Change (0.5 Point) In Redness Score Over The First 2 Hours In LDP Arms P Conjunctival Hyperemia CCLRU Scale for Reference
Summary of Positive VEGA-1 Phase 2 Results for Nyxol Eye Drops Met the primary endpoint with statistical
significance for binocular photopic near vision at 1 hour 61% Nyxol + LDP gained 15 letters (3 lines) or more vs. 28% Placebo (33% Placebo Adjusted)Met the Phase 3 co-primary endpoint vs. placebo gaining 15 letters (3 lines) near vision with
less than 5 letters of distance vision lossMet many key secondary endpointsRapid onset at 30 minDurable near vision improvement through at least 6 hoursNyxol+LDP was numerically better than each component through 2-hoursSustained significant
reduction in PD over at least 18 hours due the durability effects of NyxolNear vision efficacy seen monocularly and binocularlyAlso, efficacy data in both light and dark iris colorsFavorable safety profile for Nyxol + LDPNo serious AEsNo
systemic AEs were observed in >5% subjectsNo headaches, no browaches, and no blurry vision AEs were reportedOnly mild, transient conjunctival hyperemia observed in <5% of subjectsPositive Phase 2 results lead to advancing Phase 3
presbyopia program VEGA-1 Topline Reports (TLR) Efficacy Data In Subjects With A Favorable Safety Profile In Presbyopia With Nyxol And Low Dose Pilocarpine P

Next Steps VEGA-1 Presbyopia Presentation by Dr. Pepose at ASCRS on Sunday July 25, 2021 at 8:45amASCRS
Paper ID 76645 SPS-204 Presbyopia Correcting IOL Comparisons, New Treatments and StudiesMBCR - Level 2, Lagoon EFMIRA-2 Reversal of Mydriasis Presentation by Dr. Pepose at ASCRS on Monday July 26, 2021 at 4:25pmASCRS Paper ID 76599 SPS-316
Corneal Diagnostic StudiesMBCR - Level 2, Lagoon EF Ocuphire Plans To Present Full Results At ASCRS In July And Move Into Phase 3 P Advance into Phase 3 Presbyopia Registration Trials in 2022 Towards a Potential NDA in 2023

Presbyopia Market Opportunity

Presbyopia – Chronic Opportunity Lens loses ability to change shape when viewing objects up close as we
ageDependence on reading glasses for intermittent and prolonged useGrowing need for therapies that improve, rather than hinder, quality of life Source: GlobalData Market Research Report, 2020 The Problem Aging Population Drives Demand for
Alternatives to Reading Glasses & Very Large Market P 120 M Patients No Currently Approved Drug Therapies Effectively everyone over 40 will have the problems with reading. Physician KOL Seeking Treatment Findings Patients
requesting alternative to reading glasses 40% Patients would consider an eye drop alternative 69% ~$5B Market Opportunity
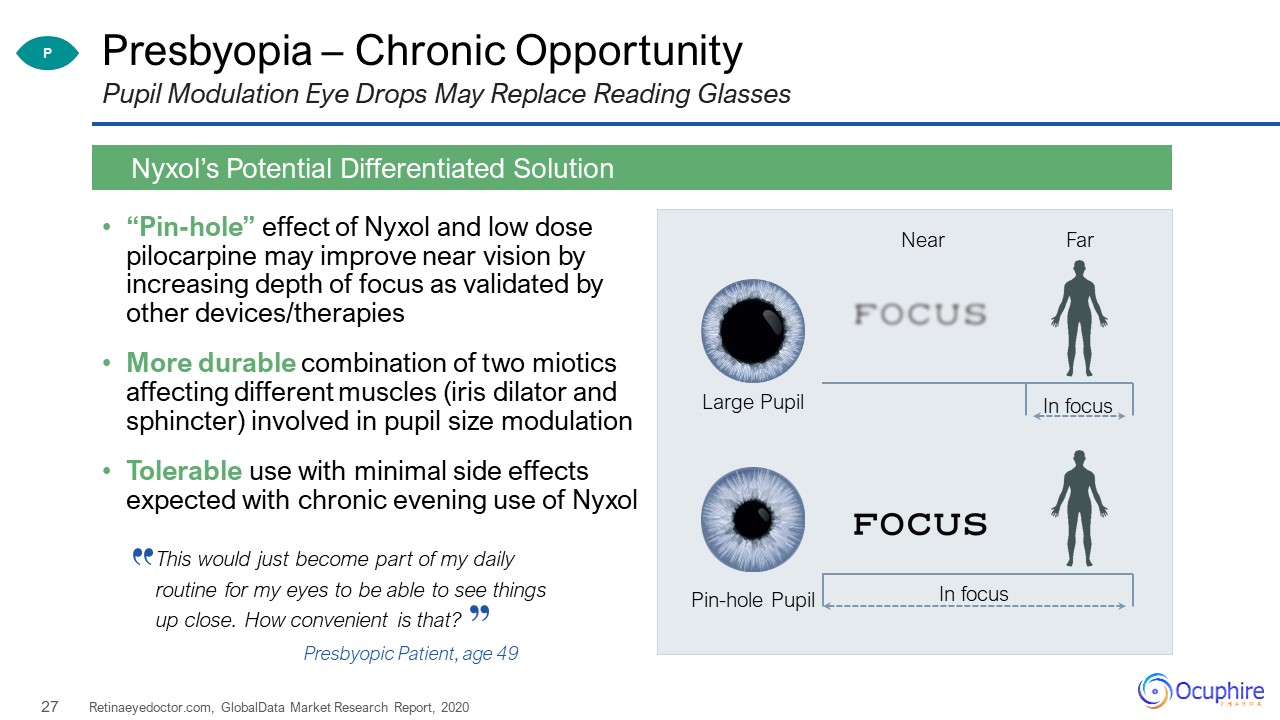
Presbyopia – Chronic Opportunity “Pin-hole” effect of Nyxol and low dose pilocarpine may improve near
vision by increasing depth of focus as validated by other devices/therapiesMore durable combination of two miotics affecting different muscles (iris dilator and sphincter) involved in pupil size modulationTolerable use with minimal side effects
expected with chronic evening use of Nyxol Retinaeyedoctor.com, GlobalData Market Research Report, 2020 Nyxol’s Potential Differentiated Solution Pupil Modulation Eye Drops May Replace Reading Glasses Large Pupil Pin-hole
Pupil Near Far In focus In focus P This would just become part of my daily routine for my eyes to be able to see things up close. How convenient is that? Presbyopic Patient, age 49
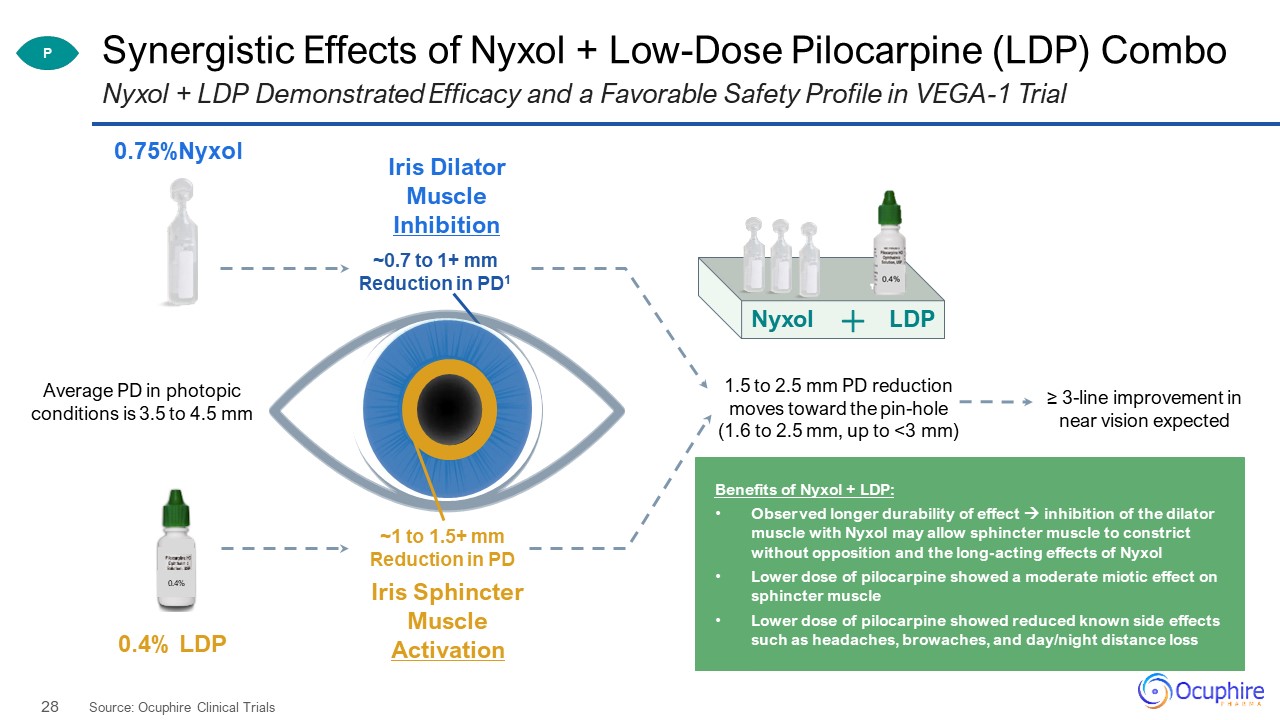
Synergistic Effects of Nyxol + Low-Dose Pilocarpine (LDP) Combo Source: Ocuphire Clinical Trials Nyxol
+ LDP Demonstrated Efficacy and a Favorable Safety Profile in VEGA-1 Trial Average PD in photopic conditions is 3.5 to 4.5 mm 1.5 to 2.5 mm PD reductionmoves toward the pin-hole (1.6 to 2.5 mm, up to <3 mm) ~0.7 to 1+ mmReduction in
PD1 ~1 to 1.5+ mmReduction in PD Nyxol LDP P Benefits of Nyxol + LDP: Observed longer durability of effect inhibition of the dilator muscle with Nyxol may allow sphincter muscle to constrict without opposition and the long-acting
effects of NyxolLower dose of pilocarpine showed a moderate miotic effect on sphincter muscleLower dose of pilocarpine showed reduced known side effects such as headaches, browaches, and day/night distance loss ≥ 3-line improvement in near
vision expected 0.4% 0.75%Nyxol 0.4% LDP Iris Dilator Muscle Inhibition Iris Sphincter Muscle Activation
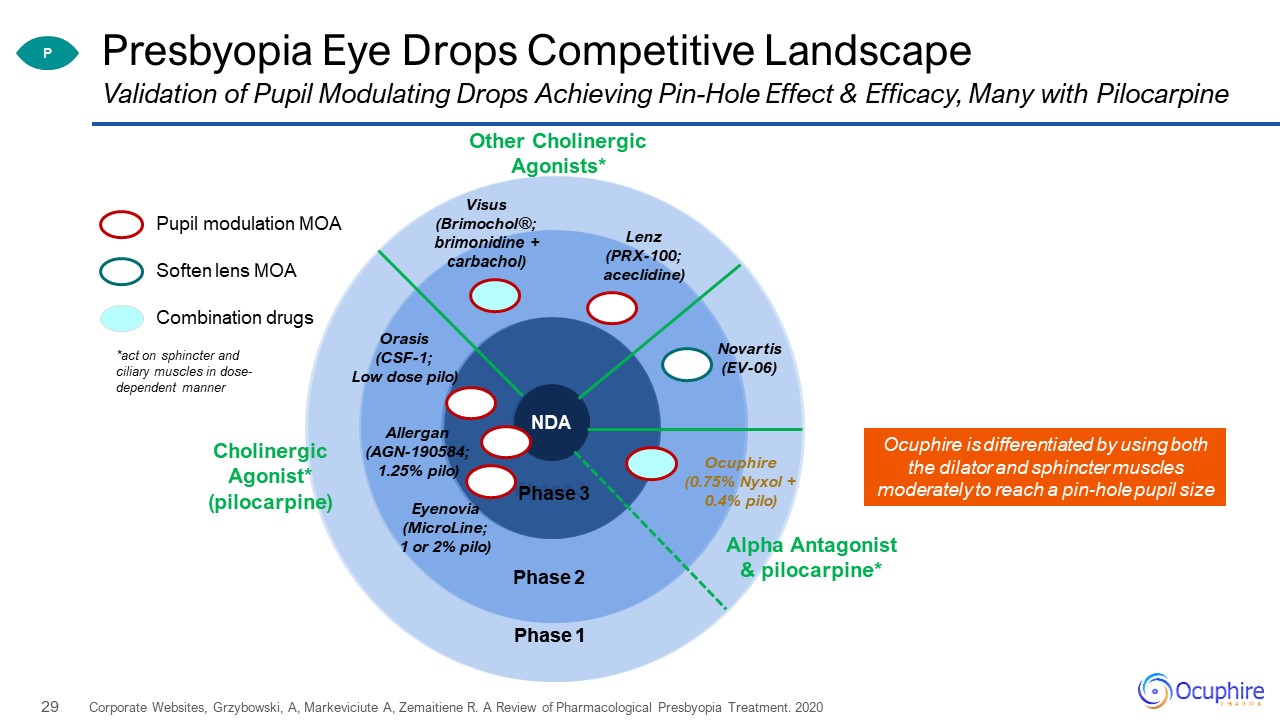
Phase 3 Phase 2 Phase 1 Presbyopia Eye Drops Competitive Landscape Corporate Websites,
Grzybowski, A, Markeviciute A, Zemaitiene R. A Review of Pharmacological Presbyopia Treatment. 2020 Validation of Pupil Modulating Drops Achieving Pin-Hole Effect & Efficacy, Many with Pilocarpine Pupil modulation MOA Combination
drugs Soften lens MOA Phase 3 Phase 2 Phase 1 Allergan(AGN-190584;1.25% pilo) Orasis(CSF-1; Low dose pilo) Ocuphire(0.75% Nyxol + 0.4% pilo) Visus(Brimochol®; brimonidine + carbachol) Other Cholinergic
Agonists* CholinergicAgonist* (pilocarpine) Lenz(PRX-100; aceclidine) Eyenovia(MicroLine;1 or 2% pilo) Novartis(EV-06) Alpha Antagonist& pilocarpine* P NDA *act on sphincter and ciliary muscles in dose-dependent
manner Ocuphire is differentiated by using both the dilator and sphincter muscles moderately to reach a pin-hole pupil size
Potential ‘Best in Class’ Presbyopia DropCompetitive Approaches Limited by Safety/Tolerability,
Durability, and Poor Distance Night Vision Nyxol + LDP Presbyopia Treatment is Differentiated: Statistically significant efficacy dataFavorable safety profileComfort and tolerabilityFast onsetLong durationMaintain good distance visual acuity
(night/day)Novel tunable pupil modulation P

Future Milestones

2021 to 2022 Ocuphire Cadence of Milestones Multiple Data Catalysts On Path To NDA(s) Ongoing
partnering discussions with leading ophthalmic companies (including European and Asian players) Completion of APX3330 LicenseARVO 2020 Presentation for MIRA-1 & ORION-1FDA EOP2 Meeting May 2020Completion of Transaction (Nasdaq: OCUP) and
concurrent $20M financingInitiate Phase 3 RM TrialInitiate Phase 3 NVD TrialComplete Nyxol Market ResearchJournal Publications Enrollment of Phase 3 RM TrialInitiate Phase 2 Presbyopia TrialReport Positive Phase 3 Data for RMInitiate Phase 2
DR/DME TrialEnrollment of Phase 2 Presbyopia TrialNew Patent ClaimsClosed $15M registered direct offeringReport Positive Phase 2 Data for Presbyopia ASCRS 2021 Presentation for MIRA-2 & VEGA-1Initiate 2nd P3 RM and Pediatric RM trial for
NDA Enrollment of Phase 3 NVD TrialReport Phase 3 Data for NVDEnrollment of Phase 2 DR/DME TrialIndustry Conferences & PublicationsManufacture 3xRegistration Batches for Nyxol Blow-Fill-Seal (BFS) Eye DropsComplete 6-month Rabbit Tox
Study Report 2nd Ph3 RM Trial Report Pediatric RM trial Report Phase 2 Data for DR/DMEInitiate 2 Phase 3 Presbyopia TrialsInitiate Chronic Ph3 Safety Trial (Nyxol /LDP)Complete 1 year CMC stability on 3xreg batchesSubmit Nyxol NDA filing for
RM in late 2022 Manufacture Commercial Batches of Nyxol Eye Drop 2020 1H 2021 2H 2021 2022* 2023* Report Phase 3 Data for Presbyopia TrialsPotential NDA for Nyxol in RMPotential Commercial Launch of Nyxol in USSubmit NDA filing for Nyxol
for Presbyopia in 2023 *Additional Studies for NVD and DR based on Data Readouts P

www.ocuphire.comir@ocuphire.com Q&A