EXHIBIT 99.1
Published on January 31, 2022

Ocuphire Pharma Investor R&D Day January 31, 2022

Disclosures And Forward-Looking Statements This presentation contains “forward-looking statements” within the meaning of the
Private Securities Litigation Reform Act of 1995. Such statements include, but are not limited to, statements concerning the regulatory timelines, commercial timelines, cash runway, and future clinical trials in RM, presbyopia, NVD and DR/DME,
including the potential for Nyxol to be a “best in class” presbyopia drop. These forward-looking statements are based upon the Company’s current expectations and involve assumptions that may never materialize or may prove to be incorrect. Actual
results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, including, without limitation: (i) the success and timing of regulatory submissions
and pre-clinical and clinical trials, including enrollment and data readouts; (ii) regulatory requirements or developments; (iii) changes to clinical trial designs and regulatory pathways; (iv) changes in capital resource requirements; (v) risks
related to the inability of Ocuphire to obtain sufficient additional capital to continue to advance its product candidates and its preclinical programs; (vi) legislative, regulatory, political and economic developments, (vii) changes in market
opportunities, (viii) the effects of COVID-19 on clinical programs and business operations, (ix) the success and timing of commercialization of any of Ocuphire’s product candidates and (x) the maintenance of Ocuphire’s intellectual property
rights. The foregoing review of important factors that could cause actual events to differ from expectations should not be construed as exhaustive and should be read in conjunction with statements that are included herein and elsewhere, including
the risk factors detailed in documents that have been and may be filed by the Company from time to time with the SEC. All forward-looking statements contained in this presentation speak only as of the date on which they were made. The Company
undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made. The Company makes no representation or warranty, express or implied, as to the accuracy or
completeness of the information contained in or incorporated by reference into this presentation. Nothing contained in or incorporated by reference into this presentation is, or shall be relied upon as, a promise or representation by the Company
as to the past or future. The Company assumes no responsibility for the accuracy or completeness of any such information. This presentation also contains estimates and other statistical data made by independent parties and by us relating to
market shares and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. The trademarks included herein are the property of the owners thereof
and are used for reference purposes only. Such use should not be construed as an endorsement of such products.

Ocuphire Investor R&D Day 1/31/22 Agenda & Speakers

Company Overview Presenter: Mina Sooch, CEO, Founder of Ocuphire Pharma
Ocuphire Pharma Restoring Vision and Clarity for Your Eyes, Today and Tomorrow
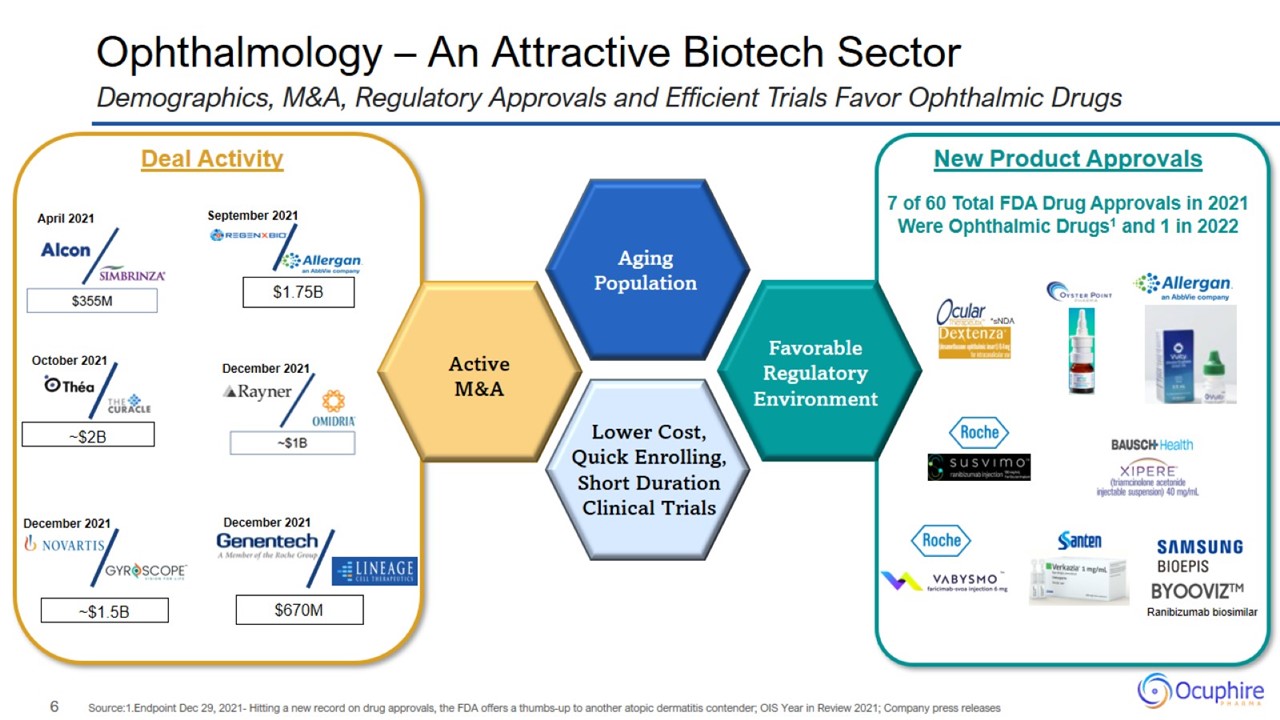
Ophthalmology – An Attractive Biotech Sector Demographics, M&A, Regulatory Approvals and Efficient Trials Favor Ophthalmic
Drugs Source:1.Endpoint Dec 29, 2021- Hitting a new record on drug approvals, the FDA offers a thumbs-up to another atopic dermatitis contender; OIS Year in Review 2021; Company press releases
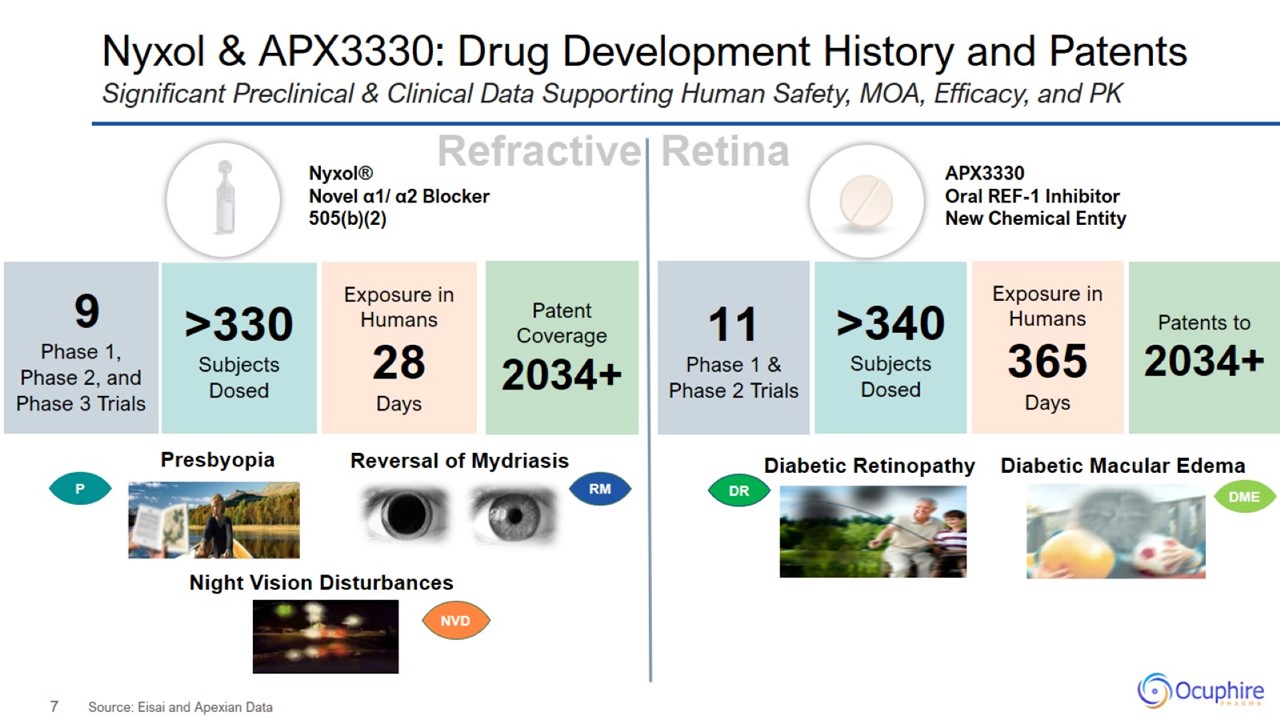
Nyxol & APX3330: Drug Development History and Patents Source: Eisai and Apexian Data Significant Preclinical & Clinical
Data Supporting Human Safety, MOA, Efficacy, and PK
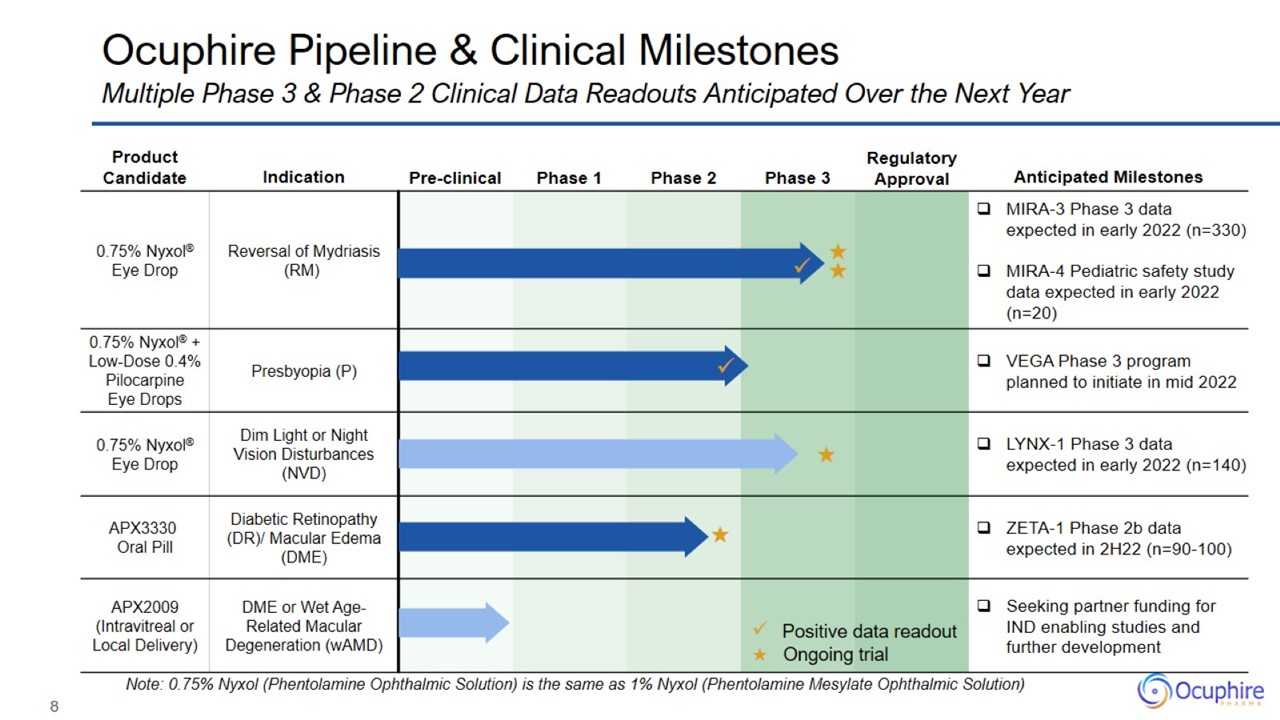
Ocuphire Pipeline & Clinical Milestones Multiple Phase 3 & Phase 2 Clinical Data Readouts Anticipated Over the Next Year

Differentiated, Late-Stage Pipeline for Front and Back of the Eye Nyxol with > 330 patients treated across 9 trials (505(b)(2)
regulatory pathway) APX3330 with > 340 patients treated across 11 trials (NCE development pathway) Nyxol and APX3330 achieved promising clinical data and favorable safety profile across multiple Phase 1, 2, and 3 trials Poised for
Commercial Success in Multiple Large Unmet Markets Addressing 4 large markets with unmet needs: RM, Presbyopia, NVD, and DR/DME Successful trial execution with 2 recent positive Phase 3 and Phase 2 data read-outs for Nyxol in RM and Nyxol + LDP
Presbyopia, respectively Stable, small-molecule drugs with commercial scalability Robust and growing IP portfolio: US and global issued thru 2034 for both assets as well as new 2039 Nyxol patent issued for presbyopia Many Catalysts in 2022
with Track Record of Execution $24.5 million cash reported at 12-31-21 sufficient for operations into 2Q 2023 Highly experienced management, Board and KOLs with broad ophthalmic and biotech drug development and commercialization success
Lower-cost, fast-enrolling, shorter-duration clinical trials Favorable, precedent regulatory environment for ophthalmic drug approval Analyst coverage by Cantor, Canaccord, Jones Trading, Alliance Global, and HCW

I. APX3330 Program Update

APX3330 Chemistry and MOA
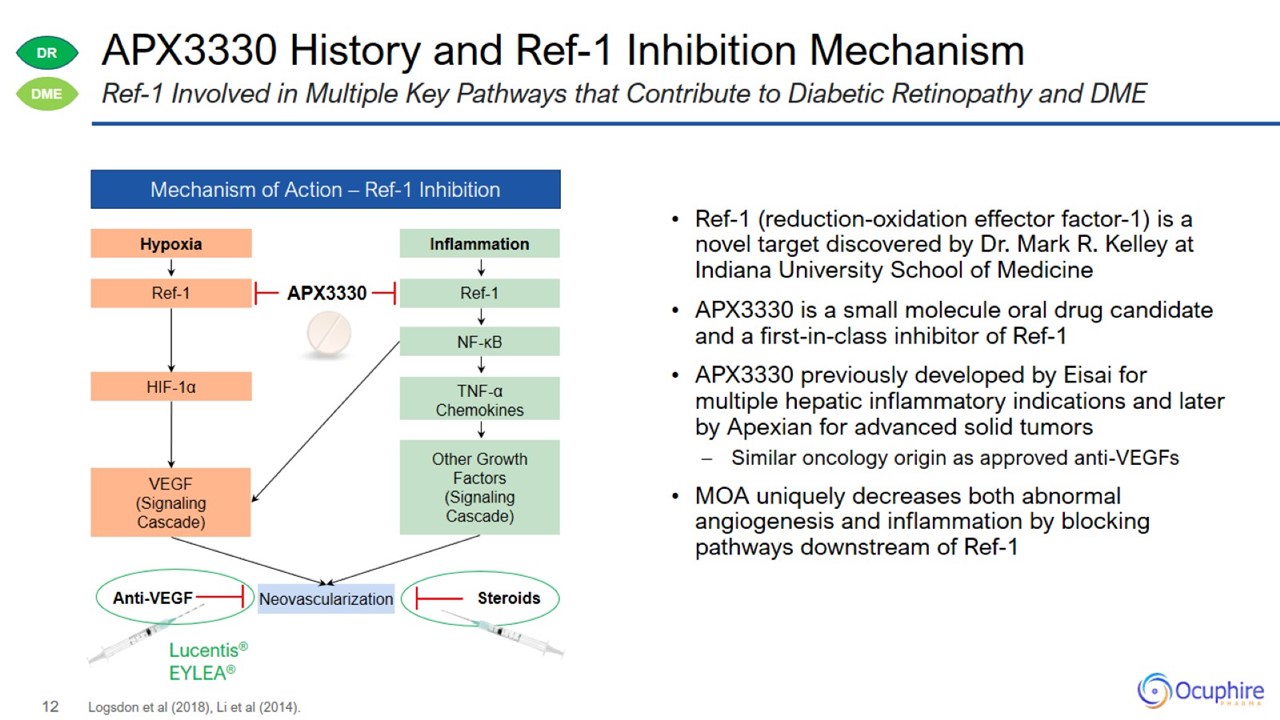
APX3330 History and Ref-1 Inhibition Mechanism Logsdon et al (2018), Li et al (2014). Ref-1 Involved in Multiple Key Pathways that Contribute to Diabetic Retinopathy and DME
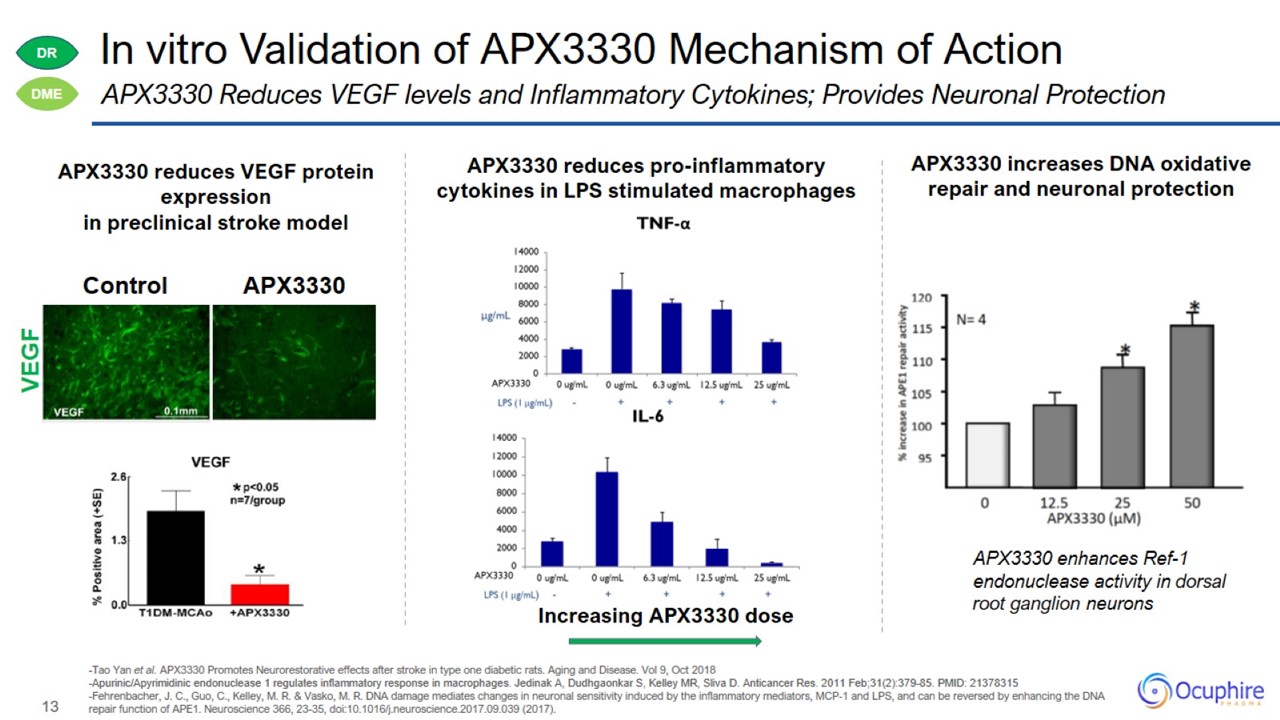
Tao Yan et al. APX3330 Promotes Neurorestorative effects after stroke in type one diabetic rats. Aging and Disease. Vol 9, Oct
2018 -Apurinic/Apyrimidinic endonuclease 1 regulates inflammatory response in macrophages. Jedinak A, Dudhgaonkar S, Kelley MR, Sliva D. Anticancer Res. 2011 Feb;31(2):379-85. PMID: 21378315 -Fehrenbacher, J. C., Guo, C., Kelley, M. R. &
Vasko, M. R. DNA damage mediates changes in neuronal sensitivity induced by the inflammatory mediators, MCP-1 and LPS, and can be reversed by enhancing the DNA repair function of APE1. Neuroscience 366, 23-35,
doi:10.1016/j.neuroscience.2017.09.039 (2017).
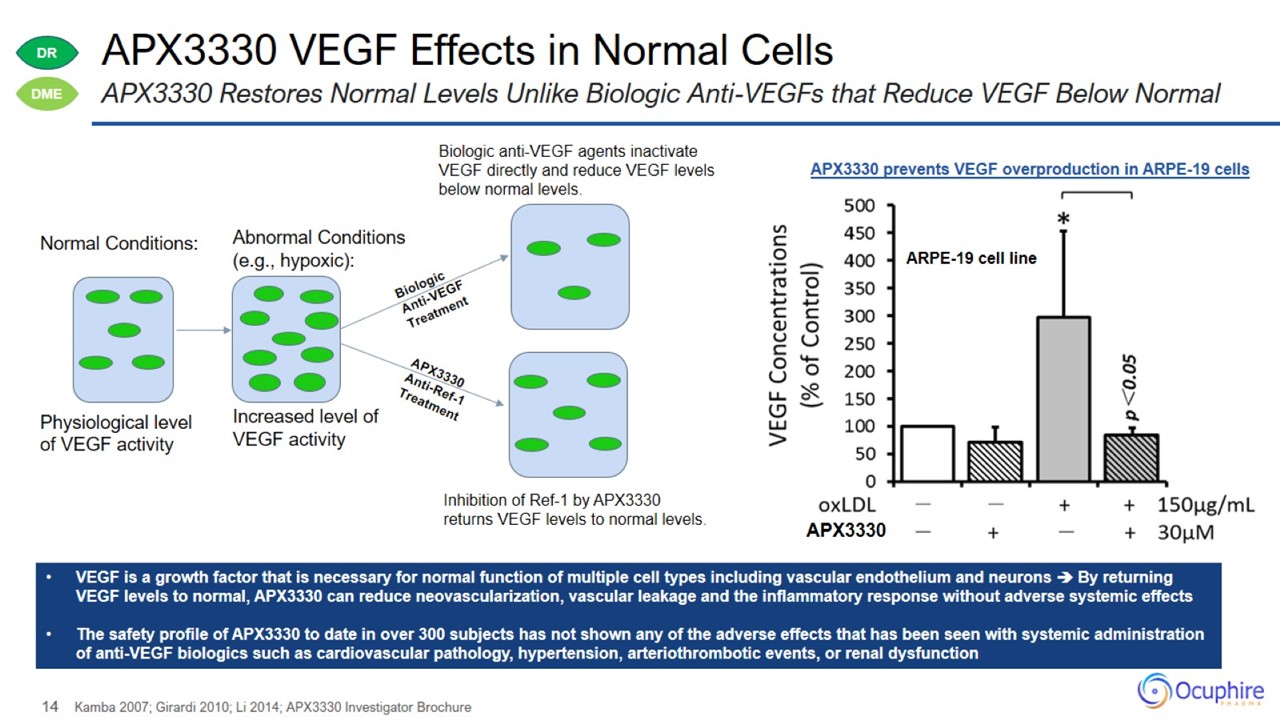
APX3330 VEGF Effects in Normal Cells Kamba 2007; Girardi 2010; Li 2014; APX3330 Investigator Brochure APX3330 Restores Normal
Levels Unlike Biologic Anti-VEGFs that Reduce VEGF Below Normal

APX3330 Preclinical & IND-Enabling Studies APX3330 Investigator Brochure Extensively Evaluated in Over 20 Studies by Large
Japanese Pharma Eisai
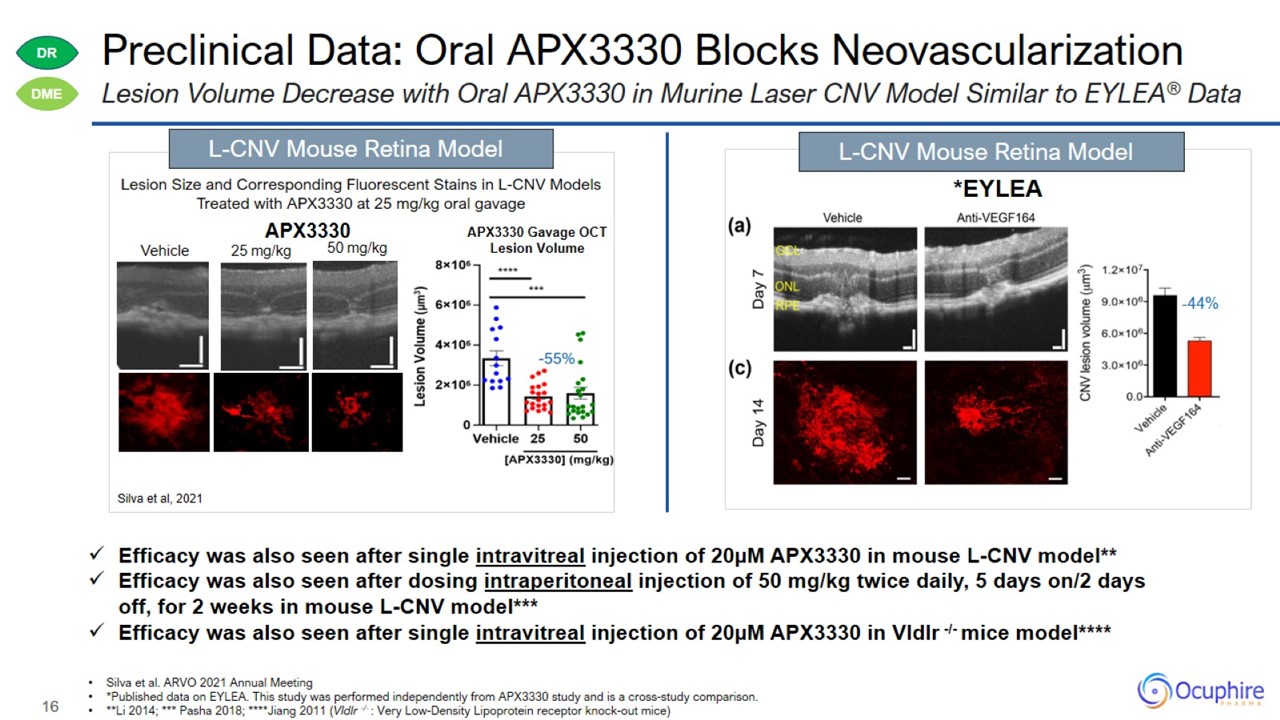
Preclinical Data: Oral APX3330 Blocks Neovascularization Silva et al. ARVO 2021 Annual Meeting *Published data on EYLEA. This
study was performed independently from APX3330 study and is a cross-study comparison. **Li 2014; *** Pasha 2018; ****Jiang 2011 (Vldlr -/- : Very Low-Density Lipoprotein receptor knock-out mice) Lesion Volume Decrease with Oral APX3330 in Murine
Laser CNV Model Similar to EYLEA® Data

APX3330 Human PK and Safety Summary
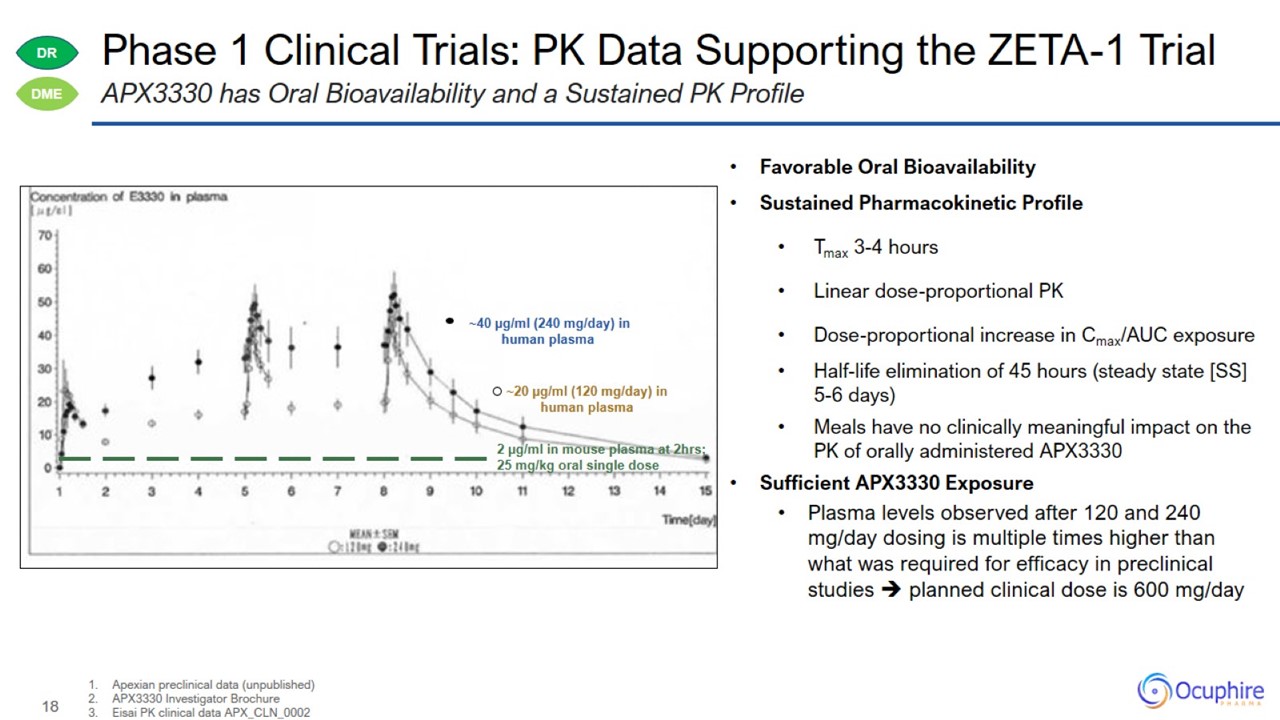
Phase 1 Clinical Trials: PK Data Supporting the ZETA-1 Trial Apexian preclinical data (unpublished) APX3330 Investigator
Brochure Eisai PK clinical data APX_CLN_0002 APX3330 has Oral Bioavailability and a Sustained PK Profile
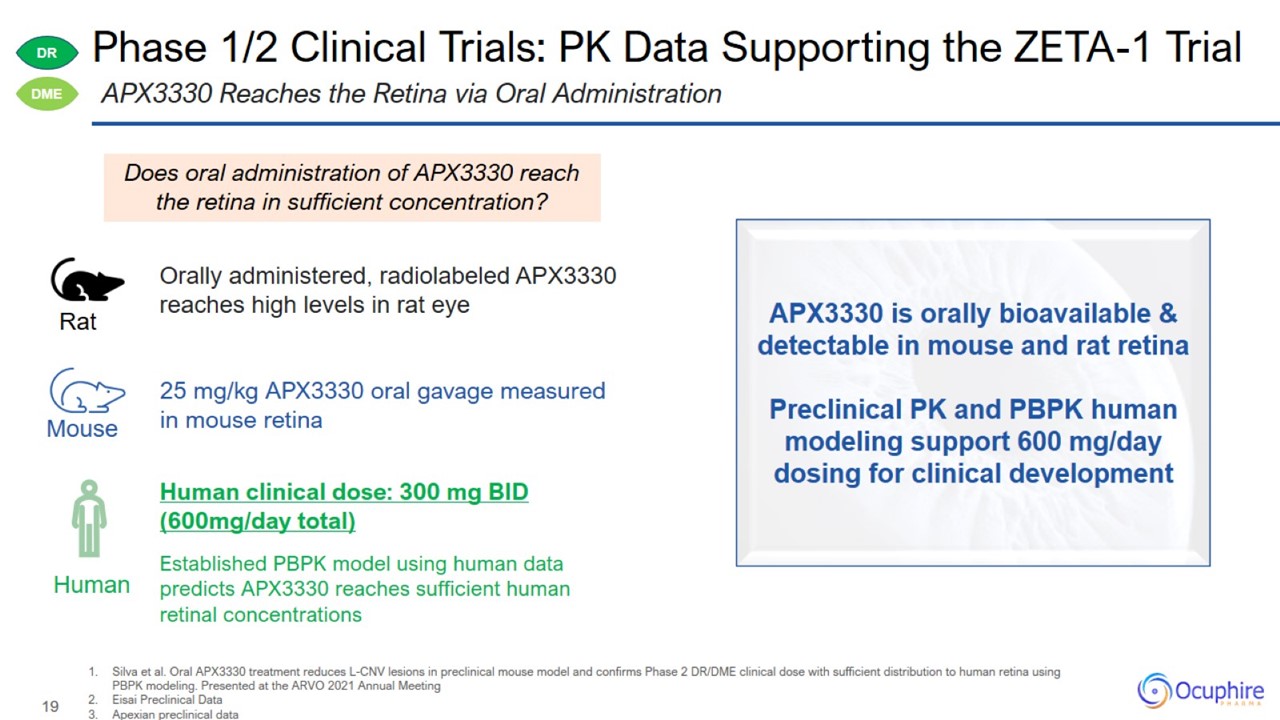
Silva et al. Oral APX3330 treatment reduces L-CNV lesions in preclinical mouse model and confirms Phase 2 DR/DME clinical dose
with sufficient distribution to human retina using PBPK modeling. Presented at the ARVO 2021 Annual Meeting Eisai Preclinical Data Apexian preclinical data APX3330 Reaches the Retina via Oral Administration
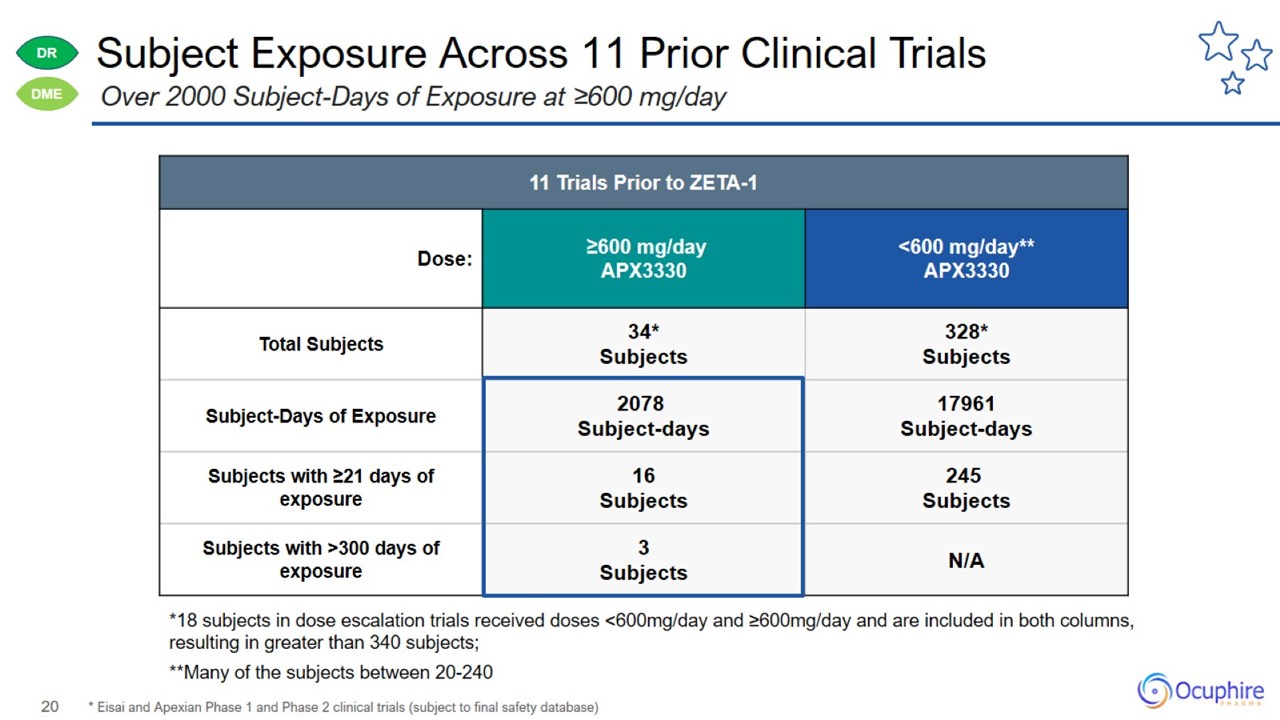
Subject Exposure Across 11 Prior Clinical Trials * Eisai and Apexian Phase 1 and Phase 2 clinical trials (subject to final safety
database) Over 2000 Subject-Days of Exposure at ≥600 mg/day
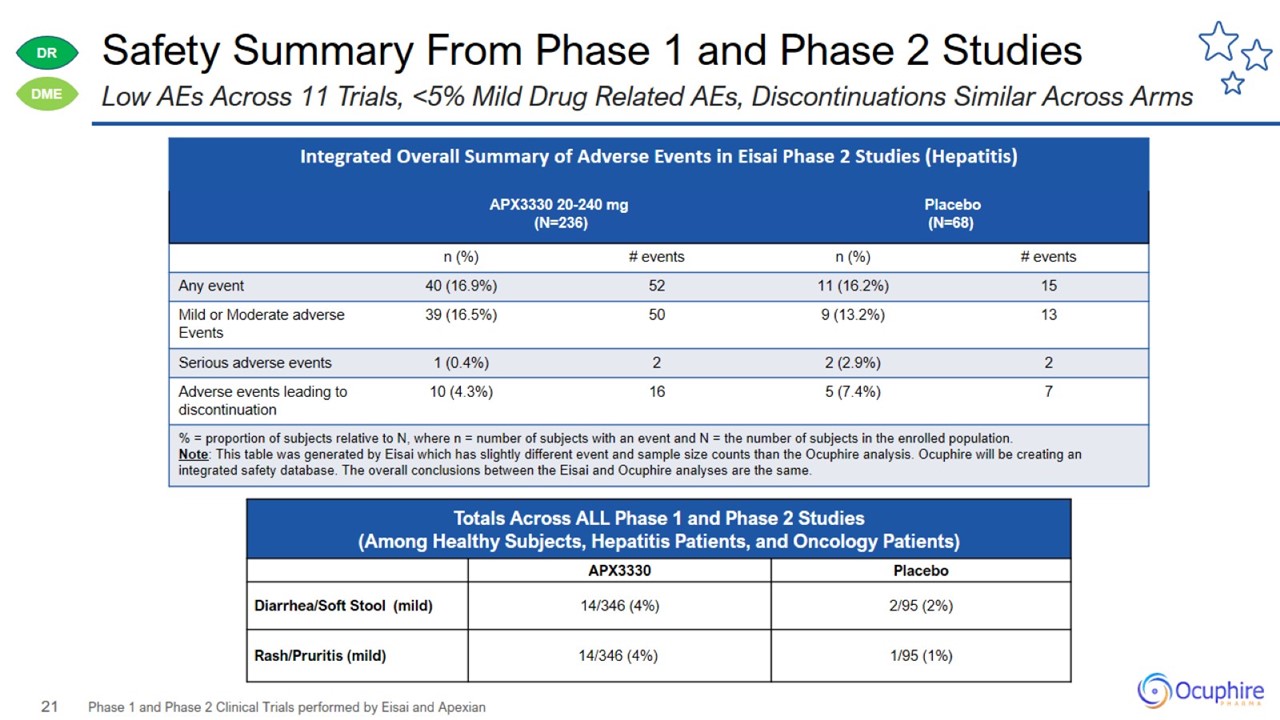
Safety Summary From Phase 1 and Phase 2 Studies Phase 1 and Phase 2 Clinical Trials performed by Eisai and Apexian
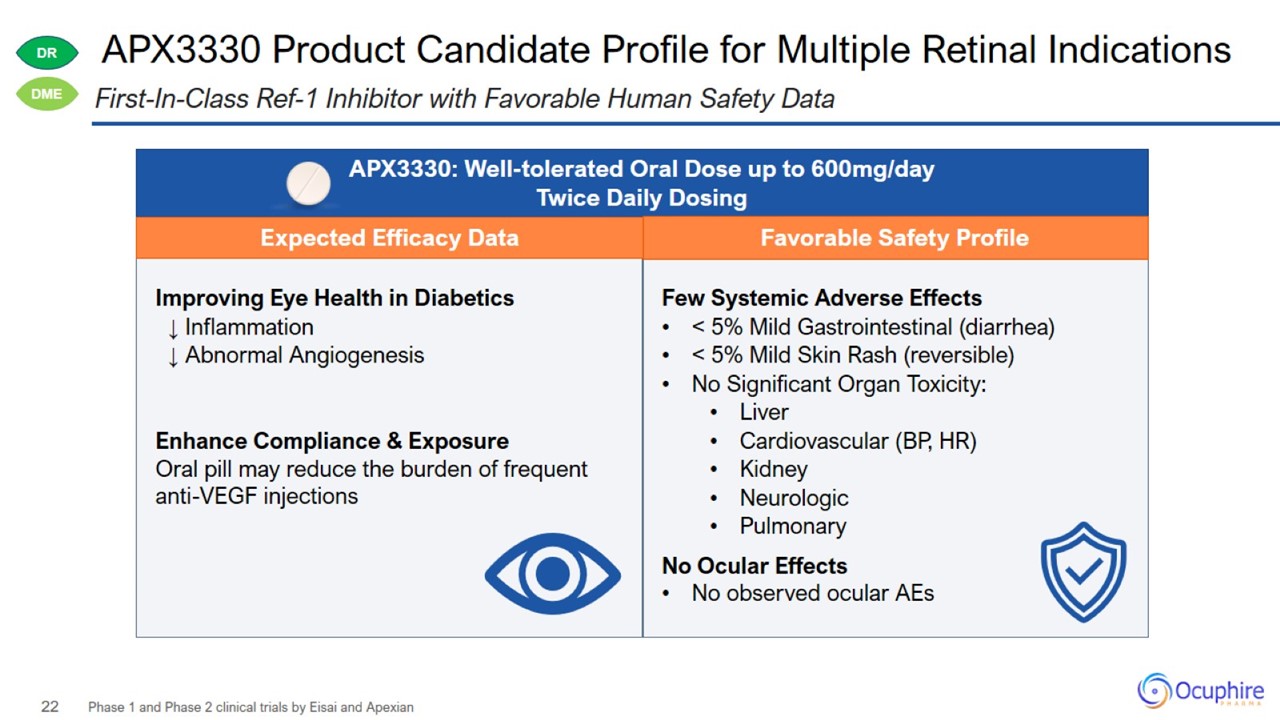
APX3330 Product Candidate Profile for Multiple Retinal Indications Phase 1 and Phase 2 clinical trials by Eisai and Apexian
First-In-Class Ref-1 Inhibitor with Favorable Human Safety Data

APX3330 Addressing Unmet Needs in Retina
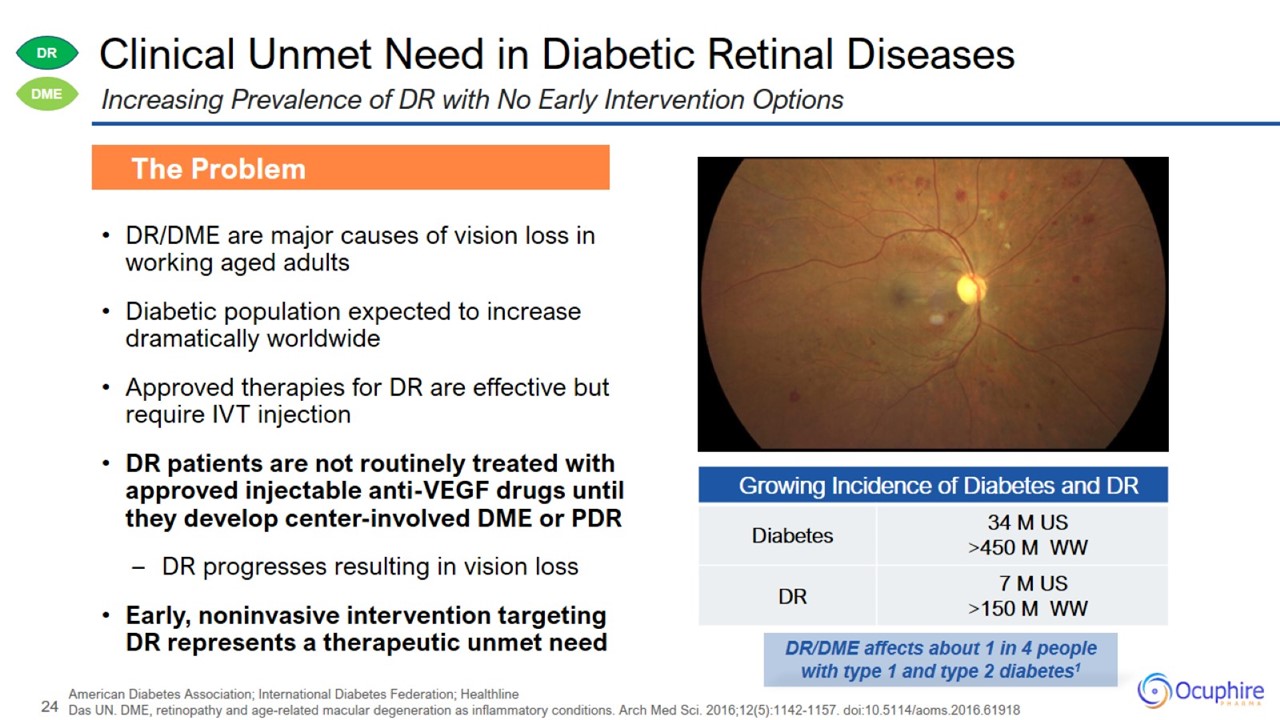
Clinical Unmet Need in Diabetic Retinal Diseases DR/DME are major causes of vision loss in working aged adults Diabetic
population expected to increase dramatically worldwide Approved therapies for DR are effective but require IVT injection DR patients are not routinely treated with approved injectable anti-VEGF drugs until they develop center-involved DME or
PDR – DR progresses resulting in vision loss • Early, noninvasive intervention targeting DR represents a therapeutic unmet need The Problem
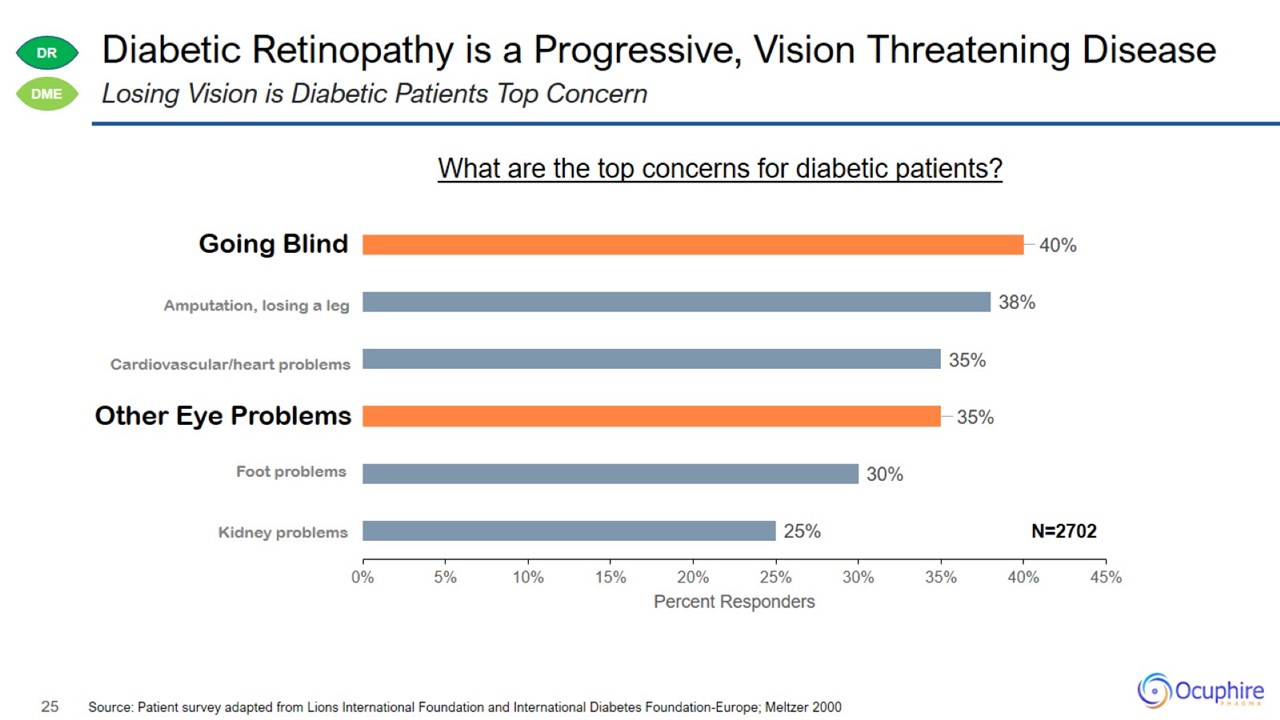
Diabetic Retinopathy is a Progressive, Vision Threatening Disease Source: Patient survey adapted from Lions International
Foundation and International Diabetes Foundation-Europe; Meltzer 2000 Losing Vision is Diabetic Patients Top Concern
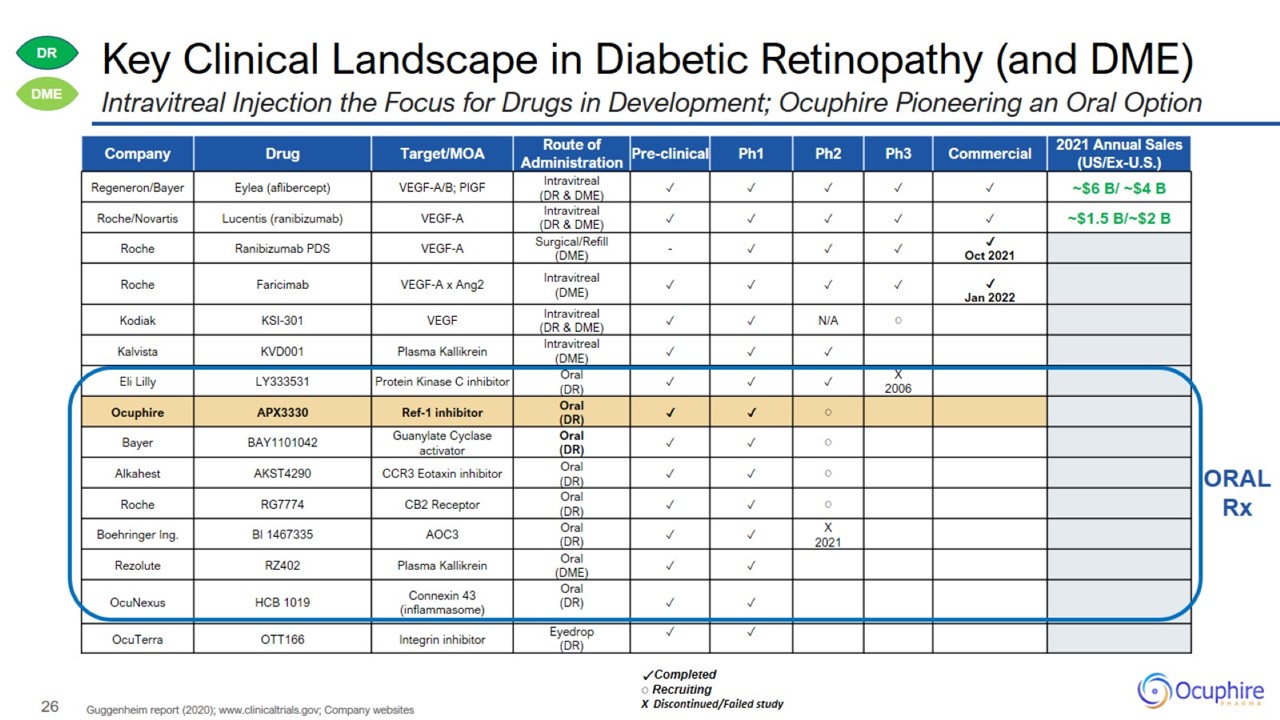
Key Clinical Landscape in Diabetic Retinopathy (and DME)

ZETA-1 Phase 2b Clinical Trial (APX3330 in DR)
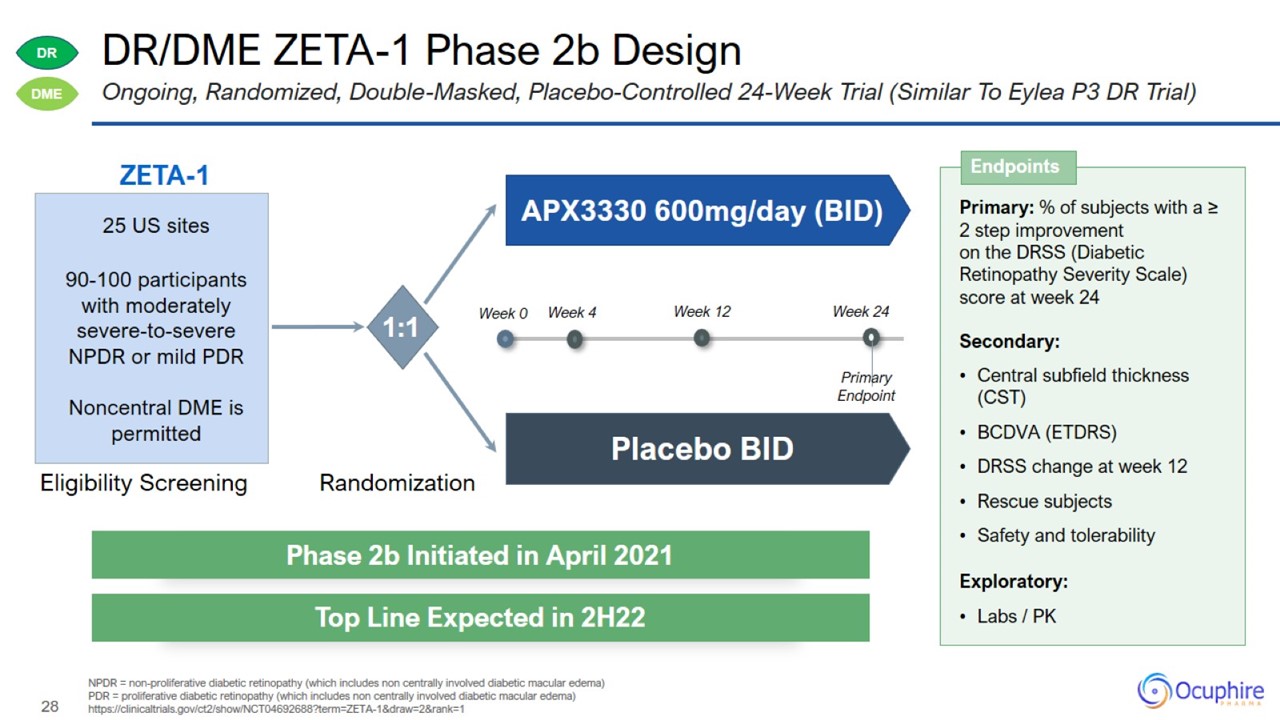
DR/DME ZETA-1 Phase 2b Design NPDR = non-proliferative diabetic retinopathy (which includes non centrally involved diabetic
macular edema) PDR = proliferative diabetic retinopathy (which includes non centrally involved diabetic macular edema) https://clinicaltrials.gov/ct2/show/NCT04692688?term=ZETA-1&draw=2&rank=1 Ongoing, Randomized, Double-Masked,
Placebo-Controlled 24-Week Trial (Similar To Eylea P3 DR Trial)
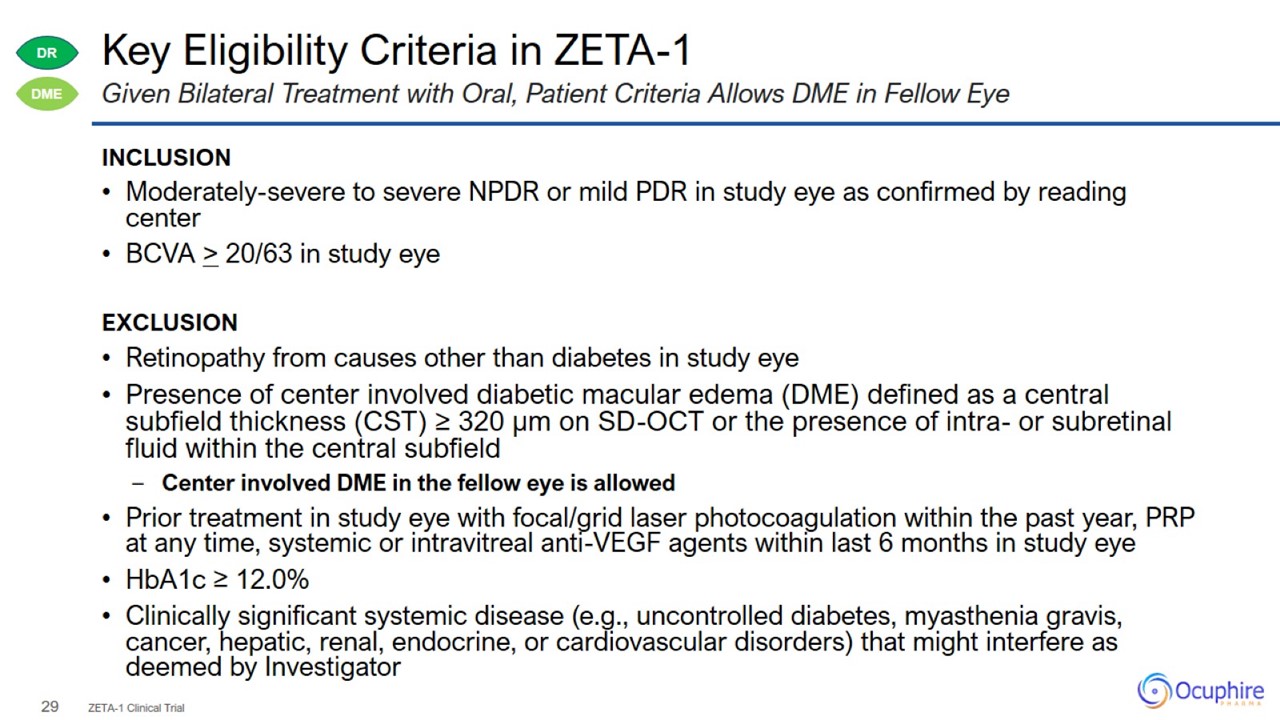
Key Eligibility Criteria in ZETA-1 INCLUSION Moderately-severe to severe NPDR or mild PDR in study eye as confirmed by reading
center BCVA > 20/63 in study eye EXCLUSION Retinopathy from causes other than diabetes in study eye Presence of center involved diabetic macular edema (DME) defined as a central
subfield thickness (CST) ≥ 320 μm on SD-OCT or the presence of intra- or subretinal fluid within the central subfield Center involved DME in the fellow eye is allowed Prior treatment in study eye with focal/grid laser photocoagulation
within the past year, PRP at any time, systemic or intravitreal anti-VEGF agents within last 6 months in study eye HbA1c ≥ 12.0% Clinically significant systemic disease (e.g., uncontrolled diabetes, myasthenia gravis, cancer, hepatic, renal,
endocrine, or cardiovascular disorders) that might interfere as deemed by Investigator ZETA-1 Clinical Trial Given Bilateral Treatment with Oral, Patient Criteria Allows DME in Fellow Eye
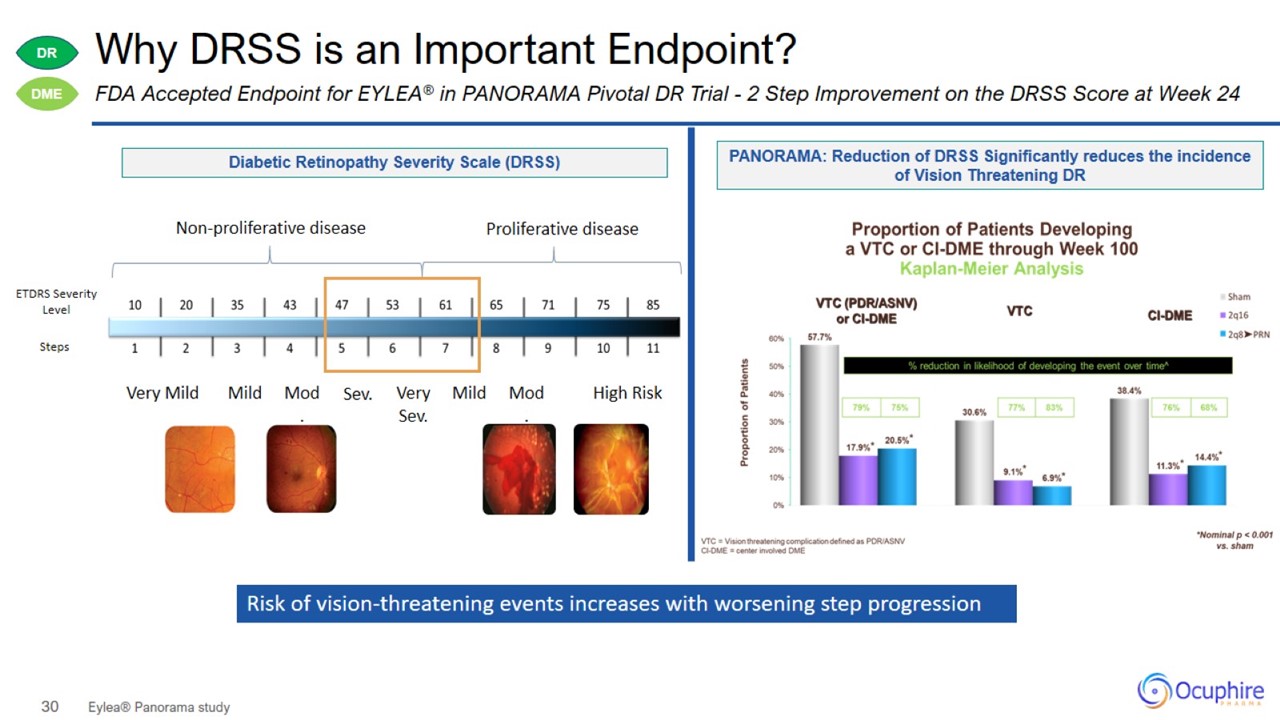
Why DRSS is an Important Endpoint? Eylea® Panorama study FDA Accepted Endpoint for EYLEA® in PANORAMA Pivotal DR Trial - 2 Step
Improvement on the DRSS Score at Week 24
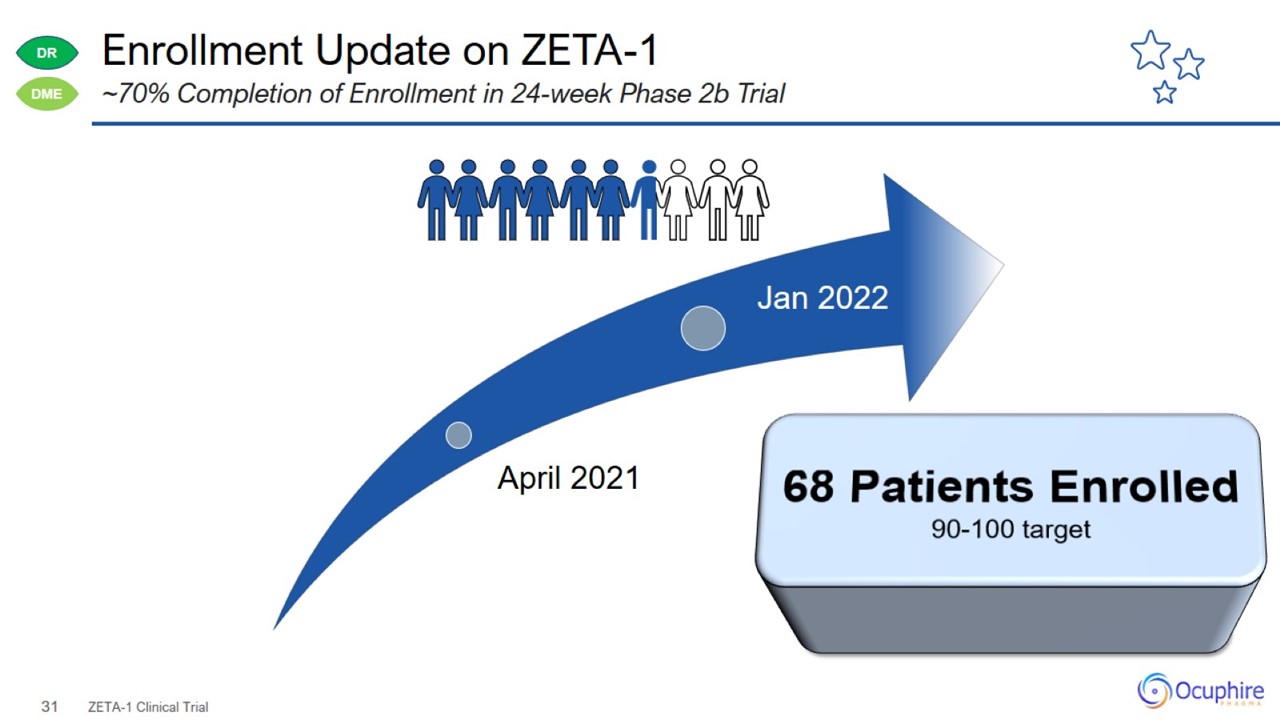
Enrollment Update on ZETA-1 ZETA-1 Clinical Trial ~70% Completion of Enrollment in 24-week Phase 2b Trial
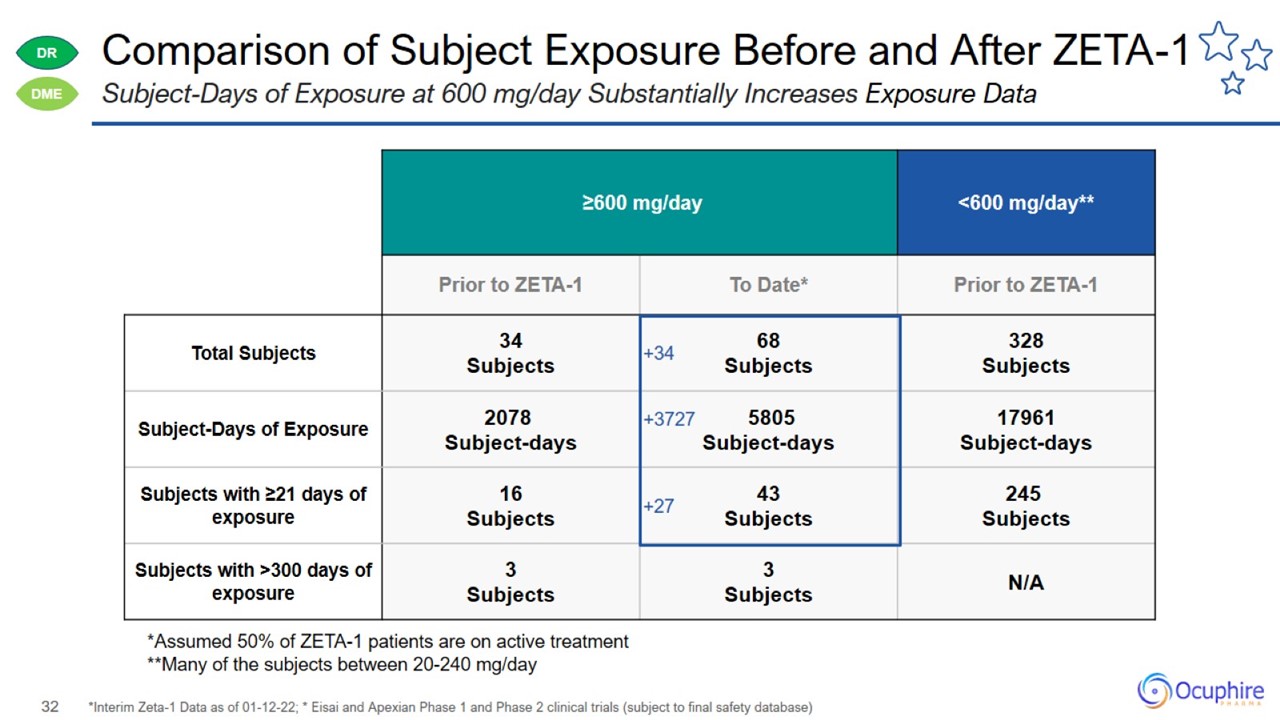
Comparison of Subject Exposure Before and After ZETA-1 *Interim Zeta-1 Data as of 01-12-22; * Eisai and Apexian Phase 1 and Phase
2 clinical trials (subject to final safety database) Subject-Days of Exposure at 600 mg/day Substantially Increases Exposure Data
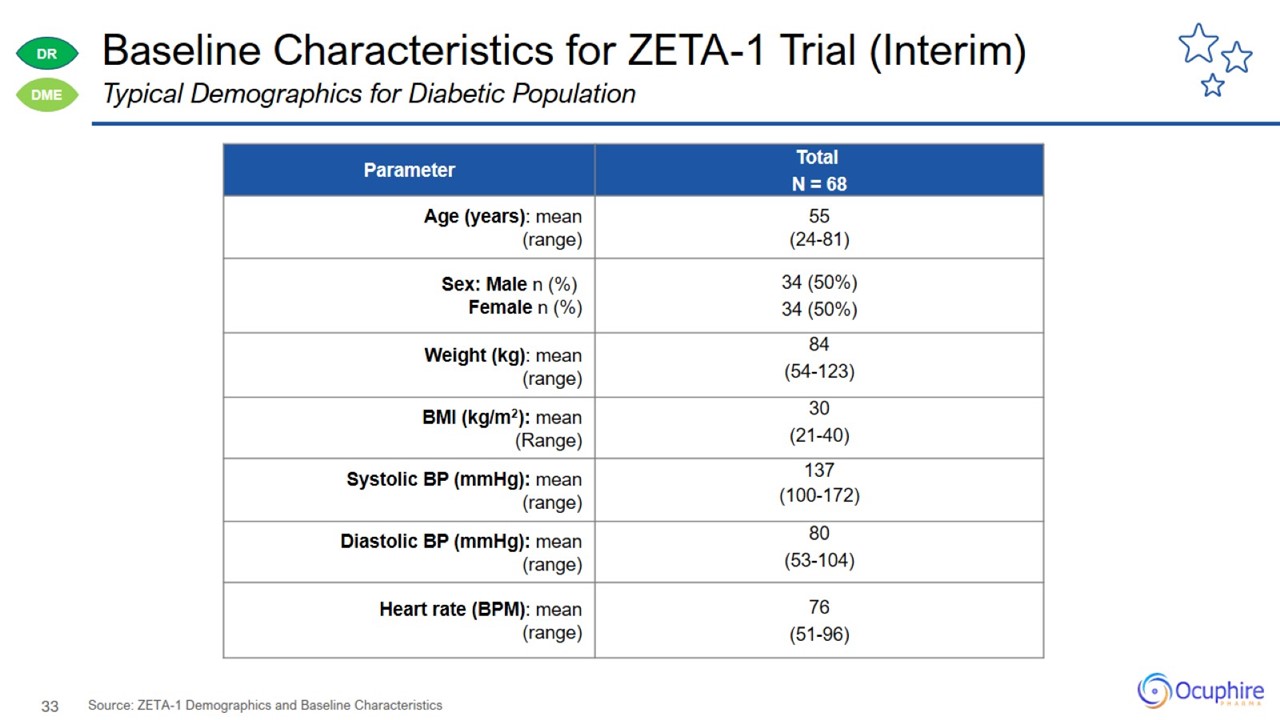
Baseline Characteristics for ZETA-1 Trial (Interim) Source: ZETA-1 Demographics and Baseline Characteristics Typical Demographics
for Diabetic Population
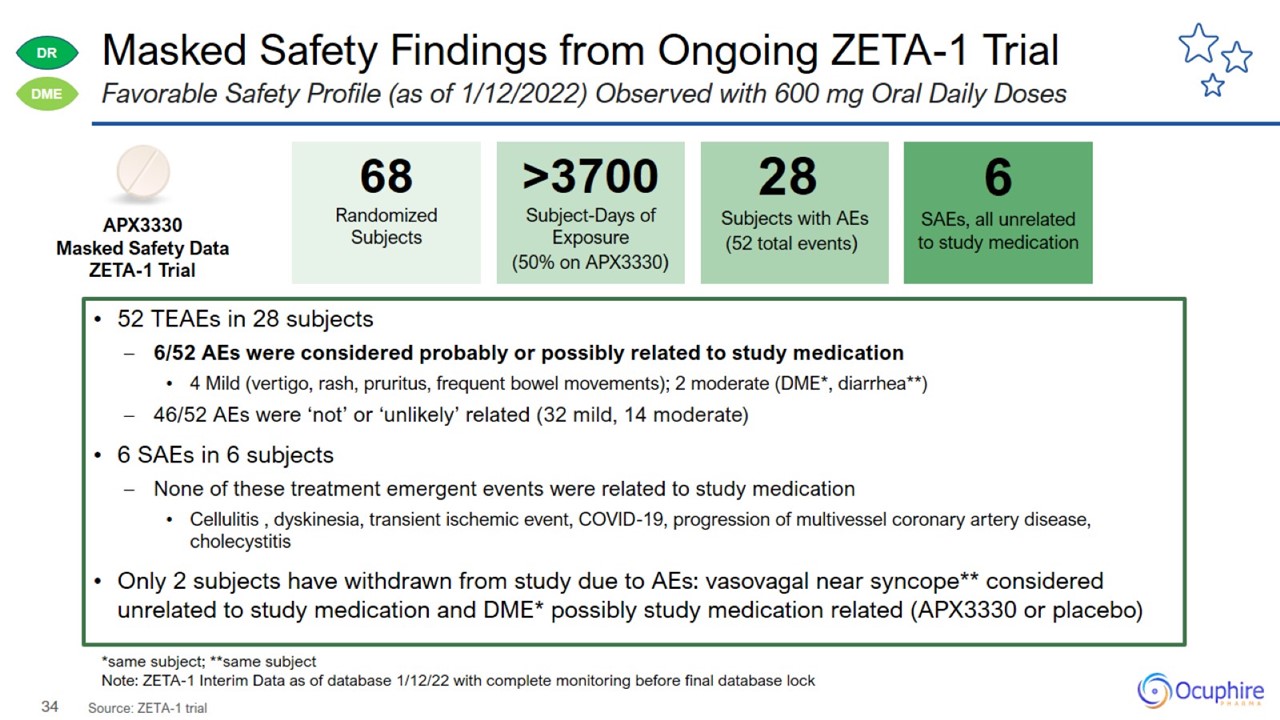
Masked Safety Findings from Ongoing ZETA-1 Trial Source: ZETA-1 trial Favorable Safety Profile (as of 1/12/2022) Observed with
600 mg Oral Daily Doses
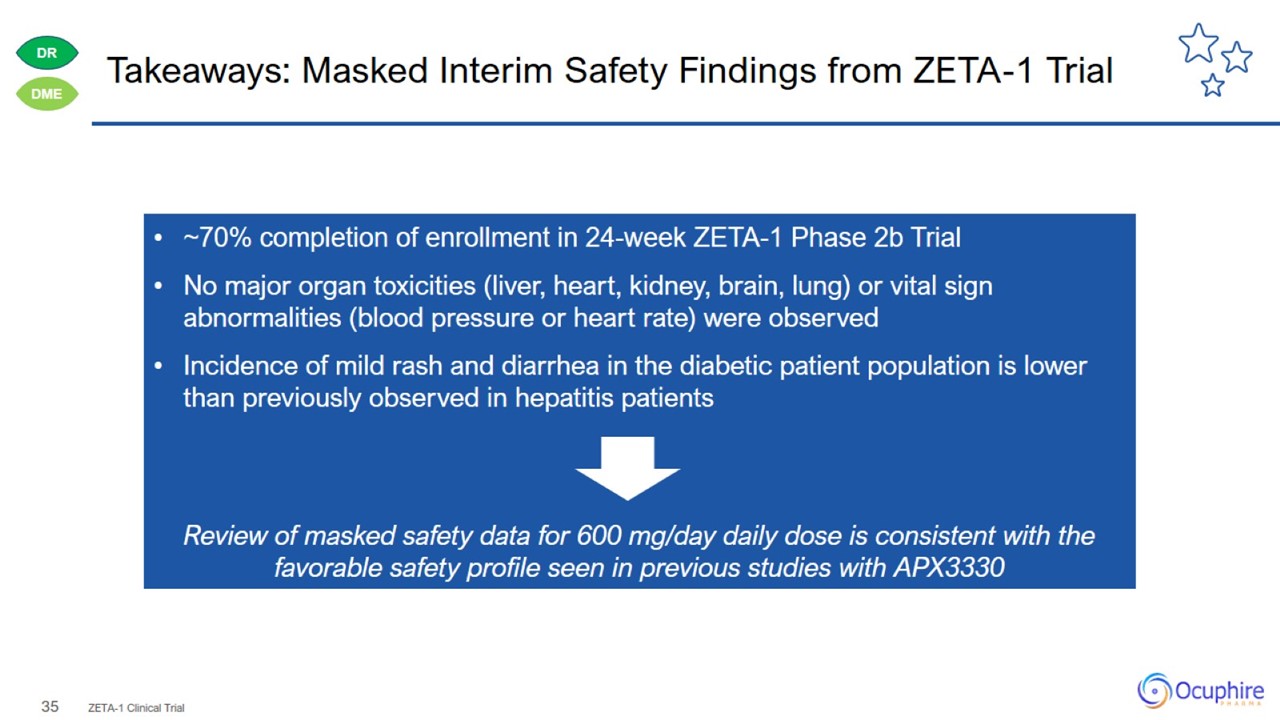
Takeaways: Masked Interim Safety Findings from ZETA-1 Trial ZETA-1 Clinical Trial
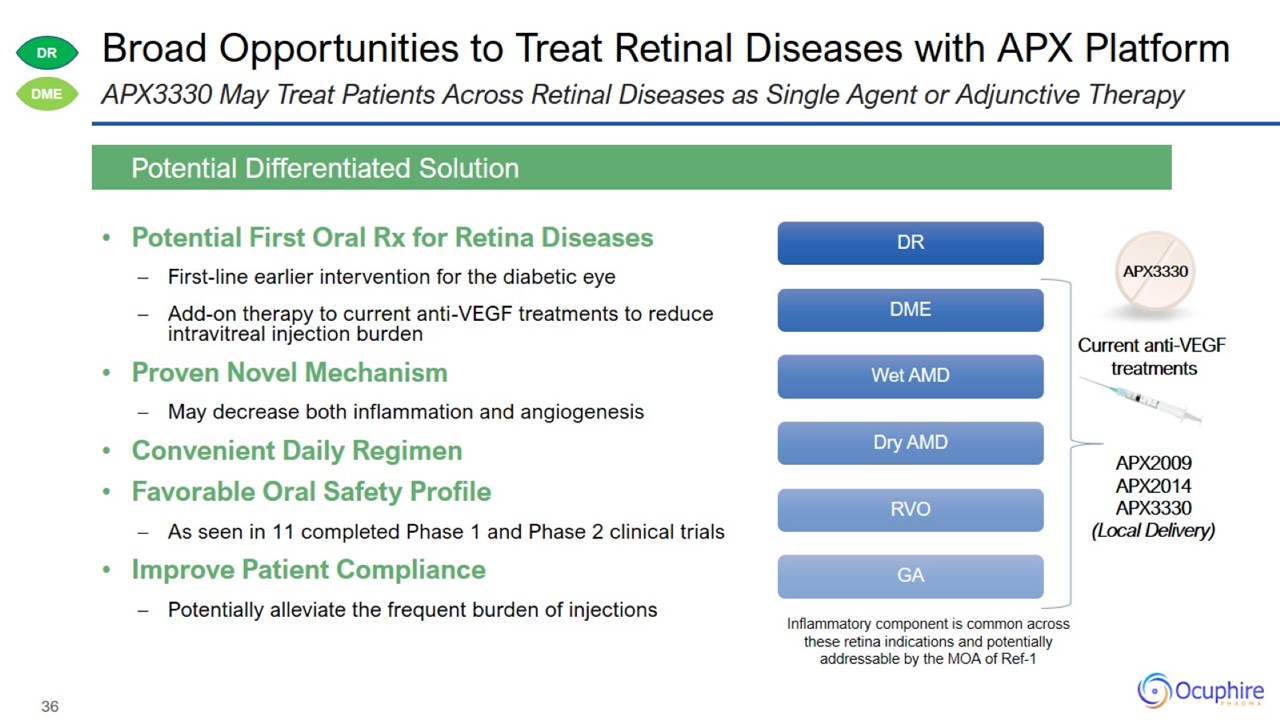
Broad Opportunities to Treat Retinal Diseases with APX Platform Potential First Oral Rx for Retina Diseases First-line earlier
intervention for the diabetic eye Add-on therapy to current anti-VEGF treatments to reduce intravitreal injection burden Proven Novel Mechanism May decrease both inflammation and angiogenesis Convenient Daily Regimen Favorable Oral Safety
Profile As seen in 11 completed Phase 1 and Phase 2 clinical trials Improve Patient Compliance Potentially alleviate the frequent burden of injections Potential Differentiated Solution APX3330 May Treat Patients Across Retinal Diseases as
Single Agent or Adjunctive Therapy
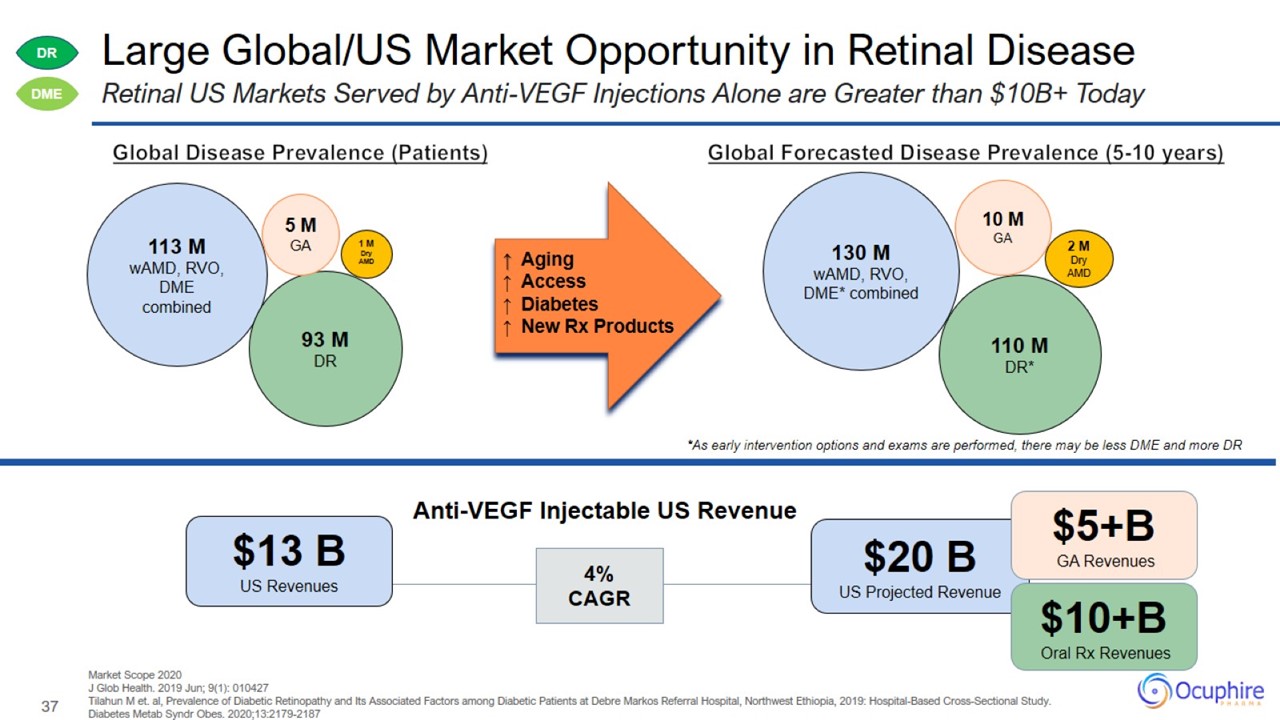
Large Global/US Market Opportunity in Retinal Disease Market Scope 2020 J Glob Health. 2019 Jun; 9(1): 010427 Tilahun M et. al,
Prevalence of Diabetic Retinopathy and Its Associated Factors among Diabetic Patients at Debre Markos Referral Hospital, Northwest Ethiopia, 2019: Hospital-Based Cross-Sectional Study. Diabetes Metab Syndr Obes. 2020;13:2179-2187 Retinal US
Markets Served by Anti-VEGF Injections Alone are Greater than $10B+ Today
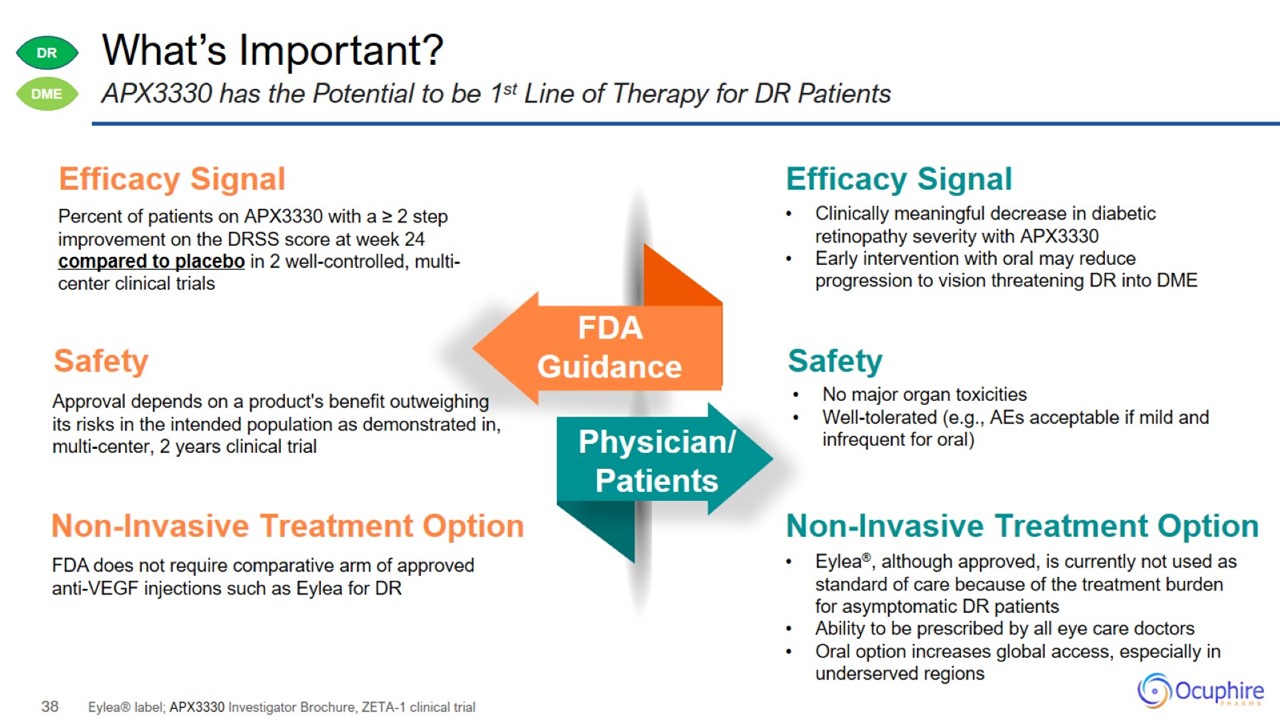
What’s Important? Eylea® label; APX3330 Investigator Brochure, ZETA-1 clinical trial APX3330 has the Potential to be 1st Line of
Therapy for DR Patients

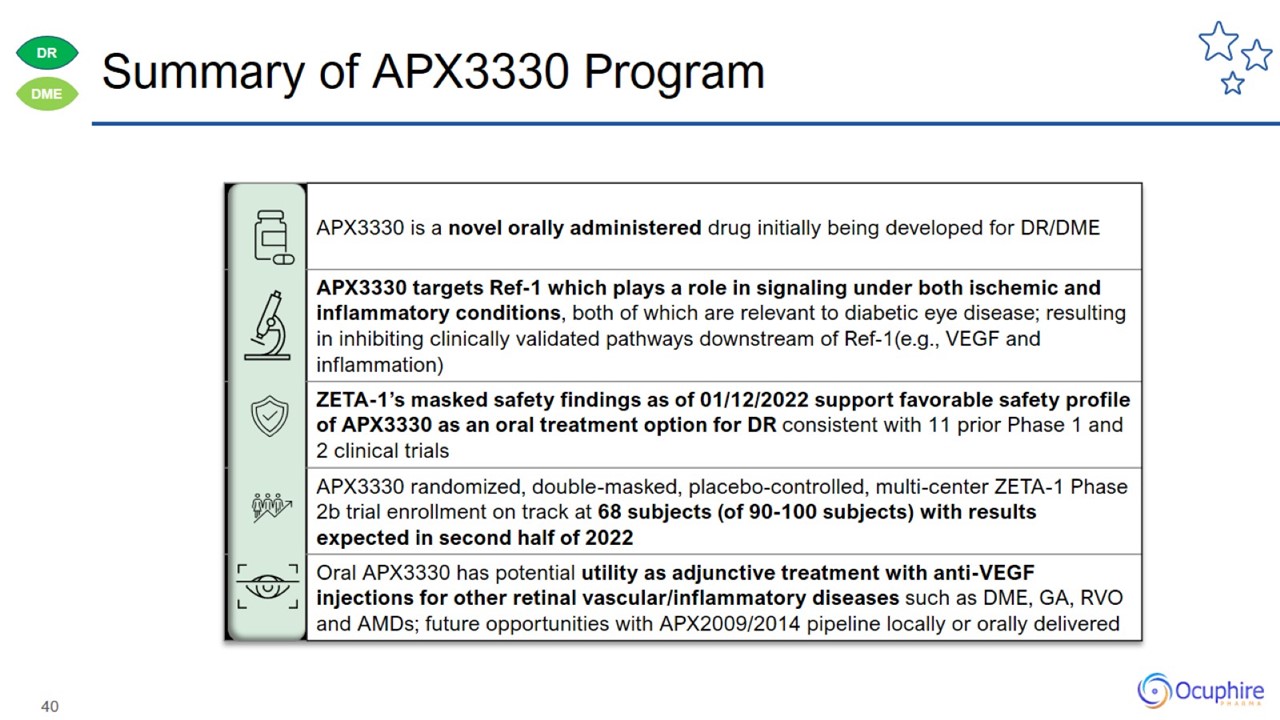
Summary of APX3330 Program

II. Nyxol Reversal of Mydriasis Overview

Reversing Dilations: Addressing an Unmet Need with α1 Blocker Nyxol
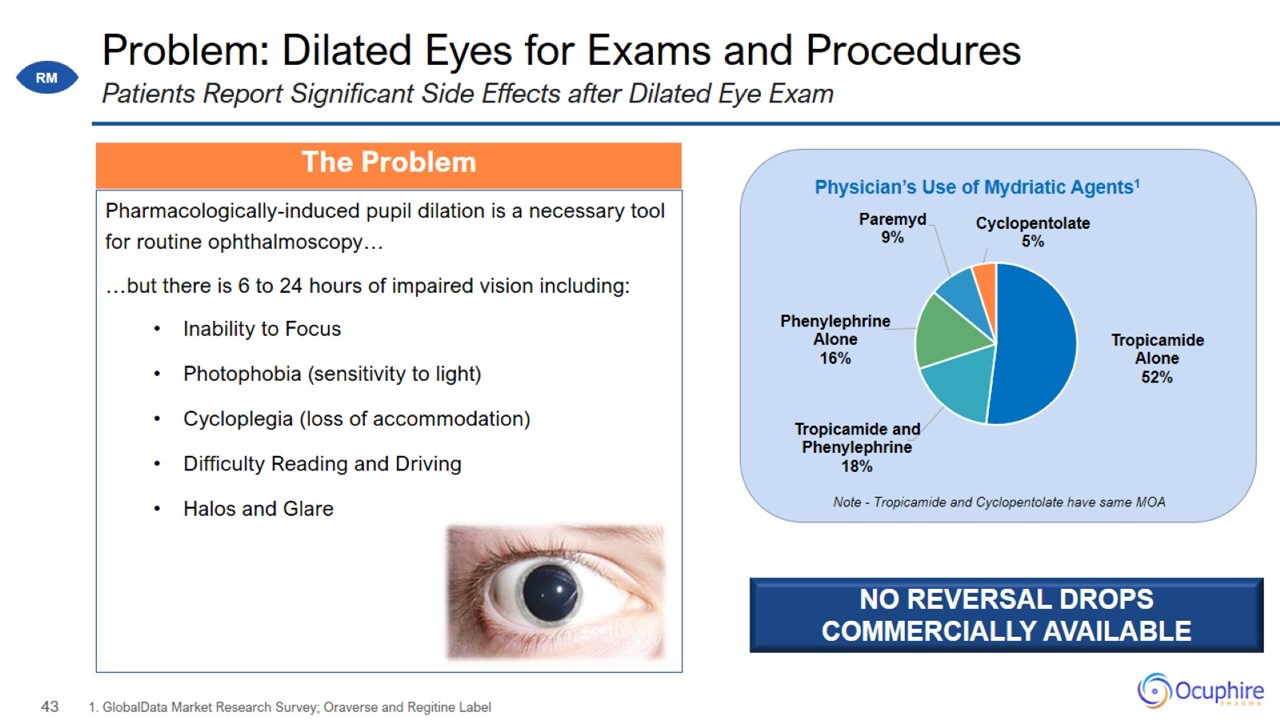
Problem: Dilated Eyes for Exams and Procedures Pharmacologically-induced pupil dilation is a necessary tool for routine
ophthalmoscopy… …but there is 6 to 24 hours of impaired vision including: Inability to Focus Photophobia (sensitivity to light) Cycloplegia (loss of accommodation) Difficulty Reading and Driving Halos and Glare 1. GlobalData Market
Research Survey; Oraverse and Regitine Label Patients Report Significant Side Effects after Dilated Eye Exam
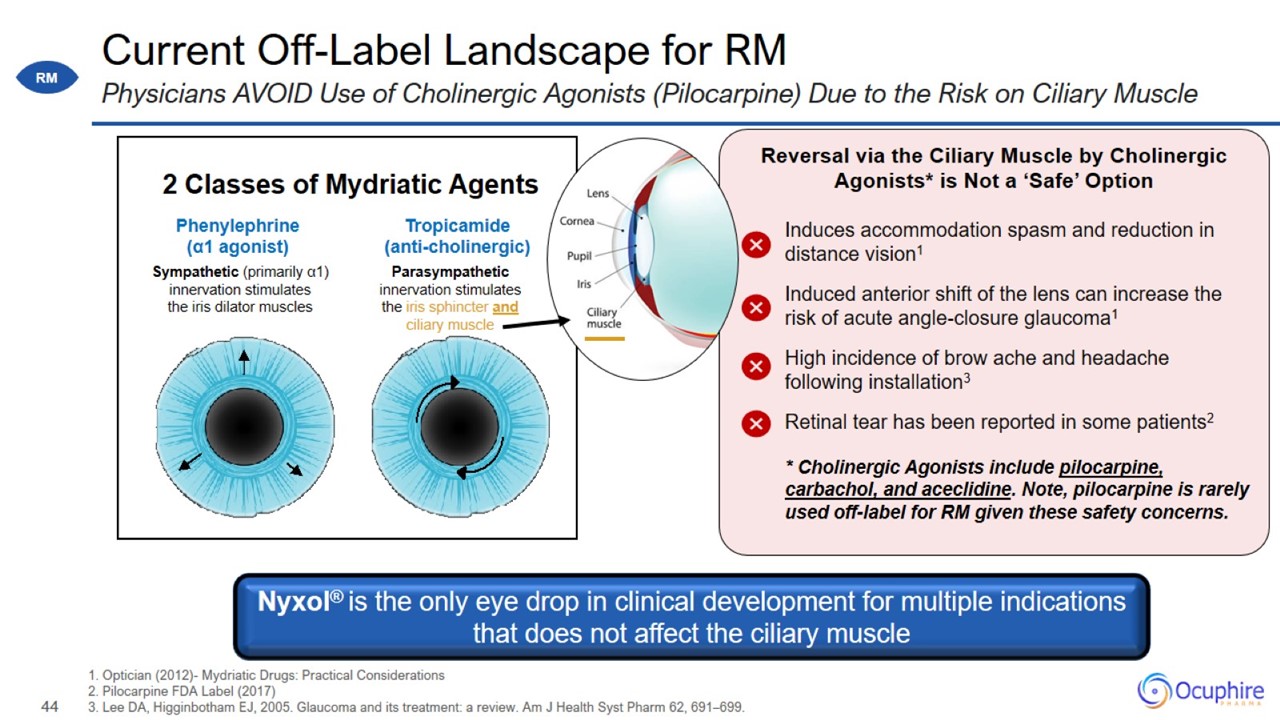
Current Off-Label Landscape for RM 1. Optician (2012)- Mydriatic Drugs: Practical Considerations 2. Pilocarpine FDA Label (2017)
3. Lee DA, Higginbotham EJ, 2005. Glaucoma and its treatment: a review. Am J Health Syst Pharm 62, 691–699. Physicians AVOID Use of Cholinergic Agonists (Pilocarpine) Due to the Risk on Ciliary Muscle
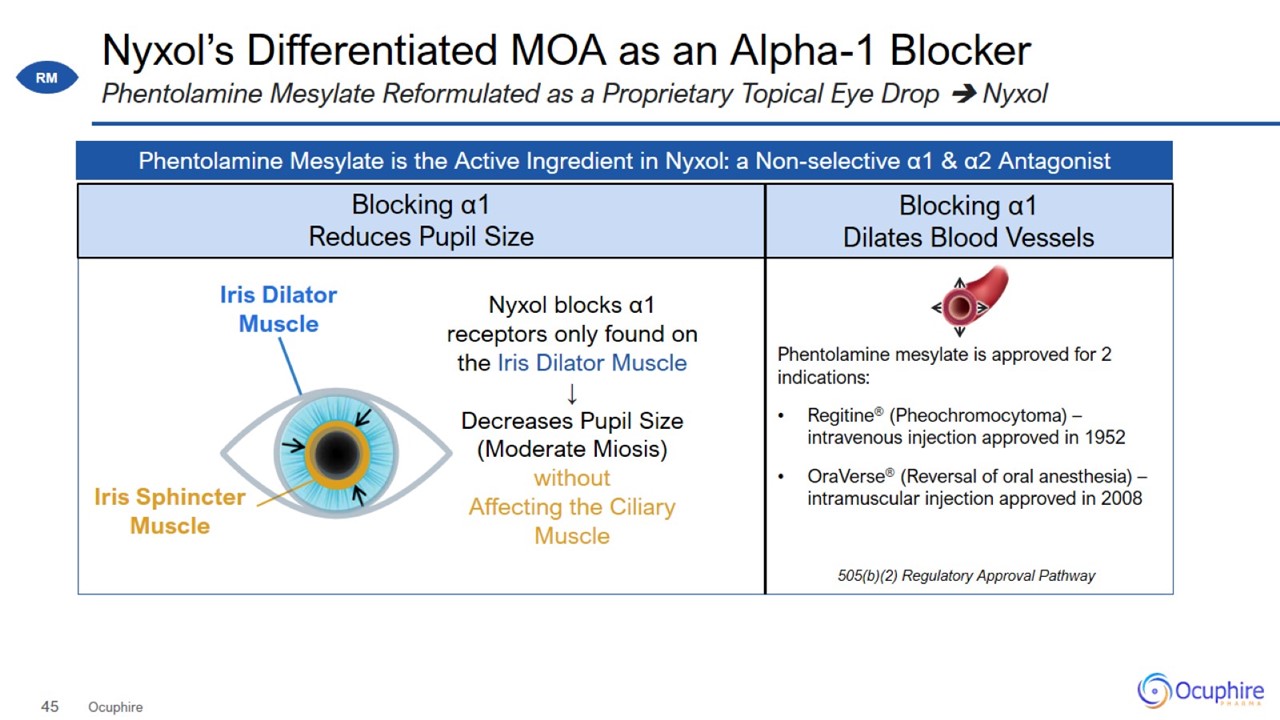
Nyxol’s Differentiated MOA as an Alpha-1 Blocker Ocuphire Phentolamine Mesylate Reformulated as a Proprietary Topical Eye Drop
Nyxol
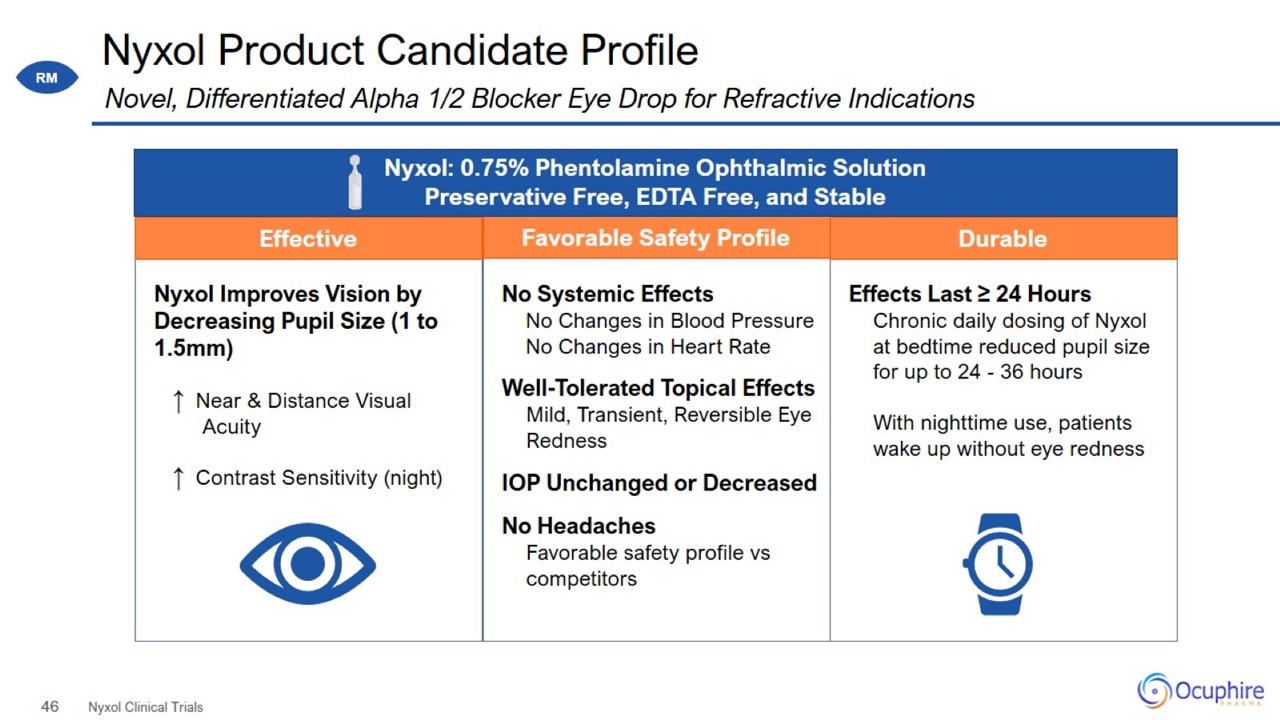
Nyxol Product Candidate Profile Nyxol Clinical Trials Novel, Differentiated Alpha 1/2 Blocker Eye Drop for Refractive Indications

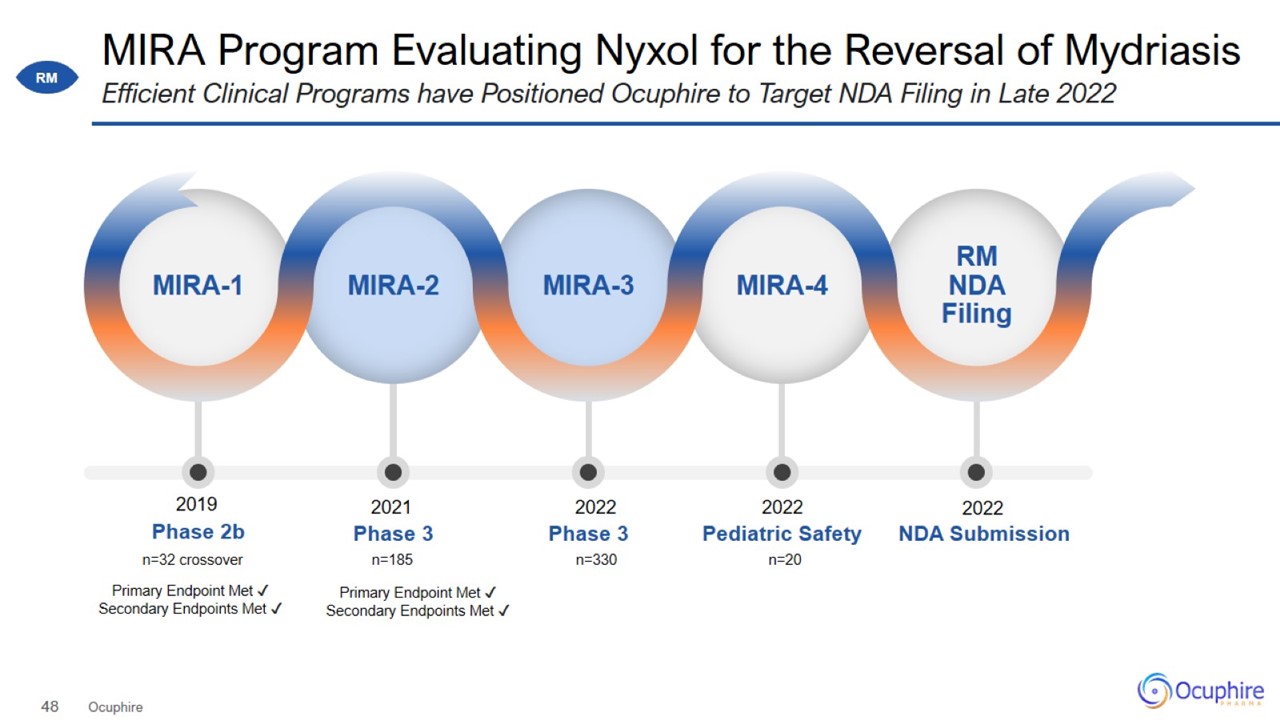
Nyxol Clinical Data for Reversing Dilations MIRA Program Evaluating Nyxol for the Reversal of Mydriasis Ocuphire Efficient
Clinical Programs have Positioned Ocuphire to Target NDA Filing in Late 2022
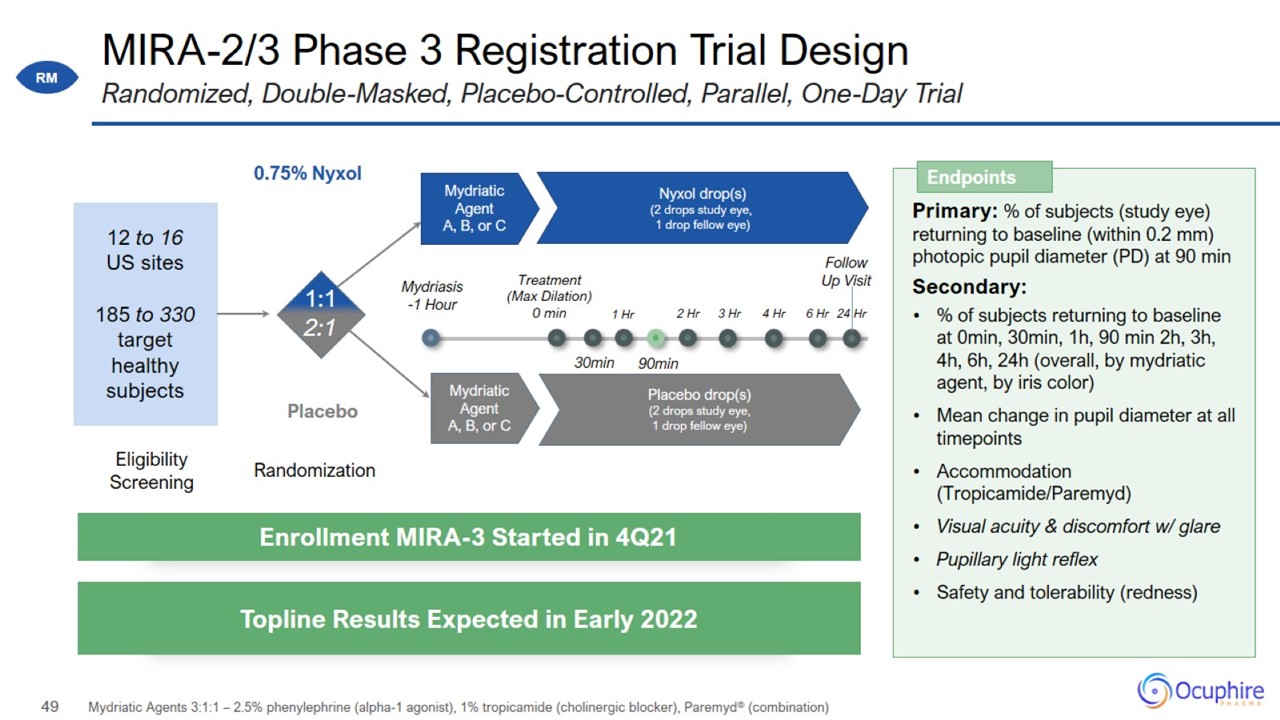
MIRA-2/3 Phase 3 Registration Trial Design Mydriatic Agents 3:1:1 – 2.5% phenylephrine (alpha-1 agonist), 1% tropicamide
(cholinergic blocker), Paremyd® (combination) Randomized, Double-Masked, Placebo-Controlled, Parallel, One-Day Trial
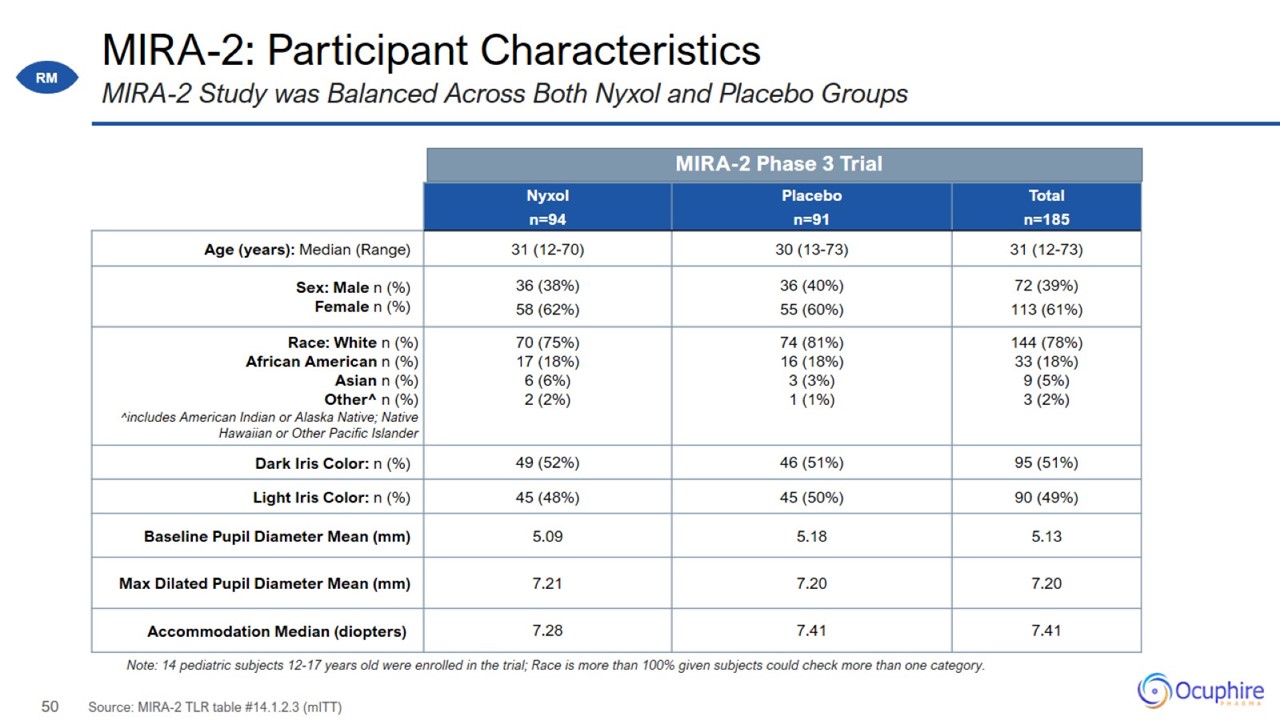
MIRA-2: Participant Characteristics Source: MIRA-2 TLR table #14.1.2.3 (mITT) MIRA-2 Study was Balanced Across Both Nyxol and
Placebo Groups
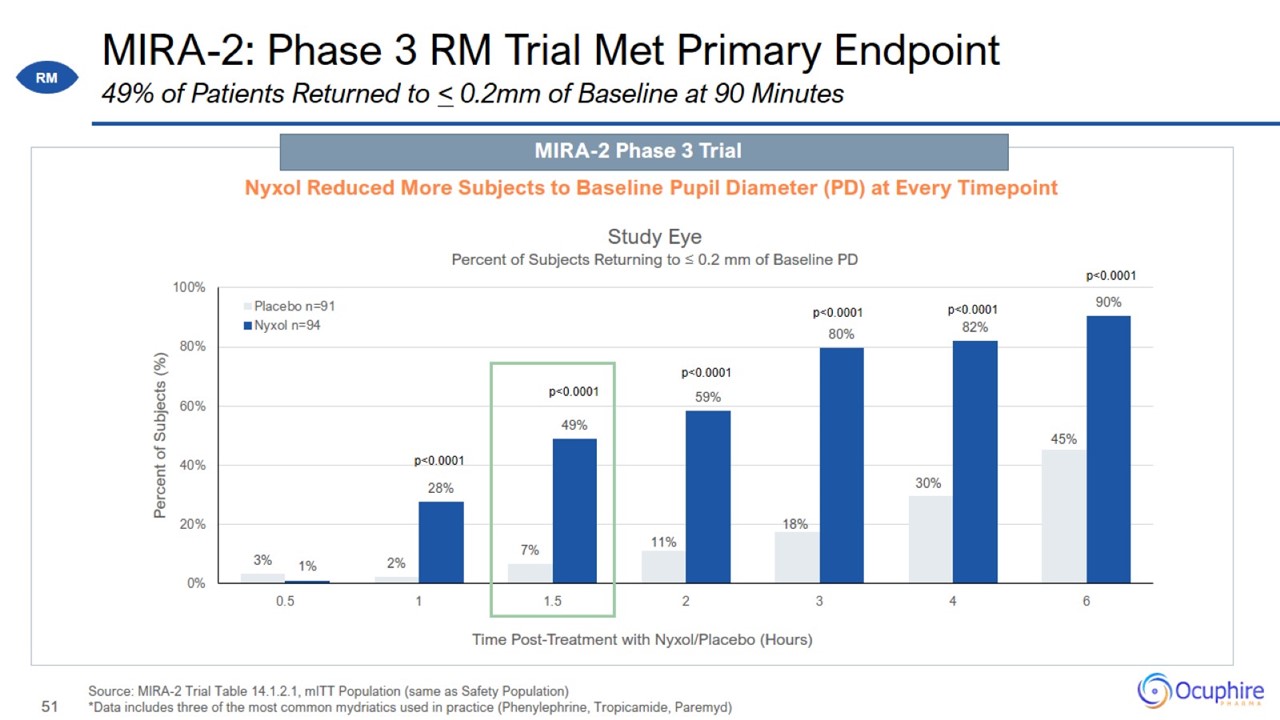
MIRA-2: Phase 3 RM Trial Met Primary Endpoint Source: MIRA-2 Trial Table 14.1.2.1, mITT Population (same as Safety Population)
*Data includes three of the most common mydriatics used in practice (Phenylephrine, Tropicamide, Paremyd) 49% of Patients Returned to < 0.2mm of Baseline at 90 Minutes
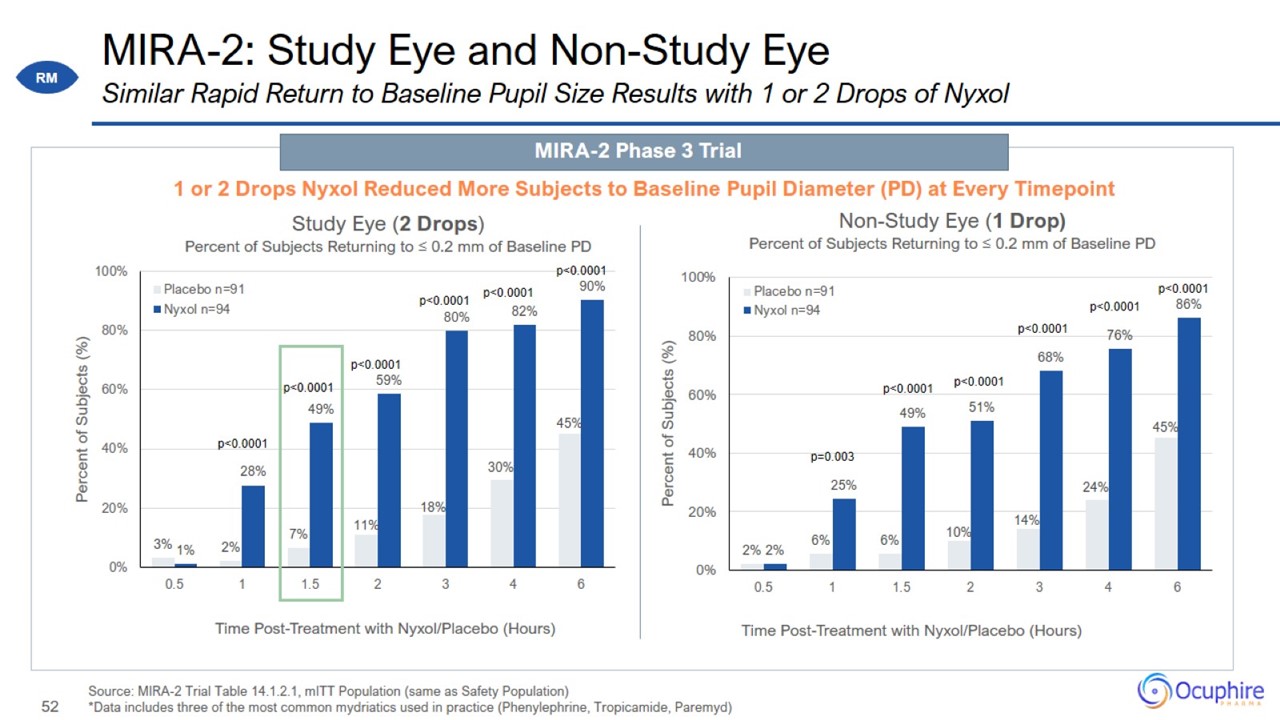
MIRA-2: Study Eye and Non-Study Eye Source: MIRA-2 Trial Table 14.1.2.1, mITT Population (same as Safety Population) *Data includes three of the most common mydriatics used
in practice (Phenylephrine, Tropicamide, Paremyd) Similar Rapid Return to Baseline Pupil Size Results with 1 or 2 Drops of Nyxol
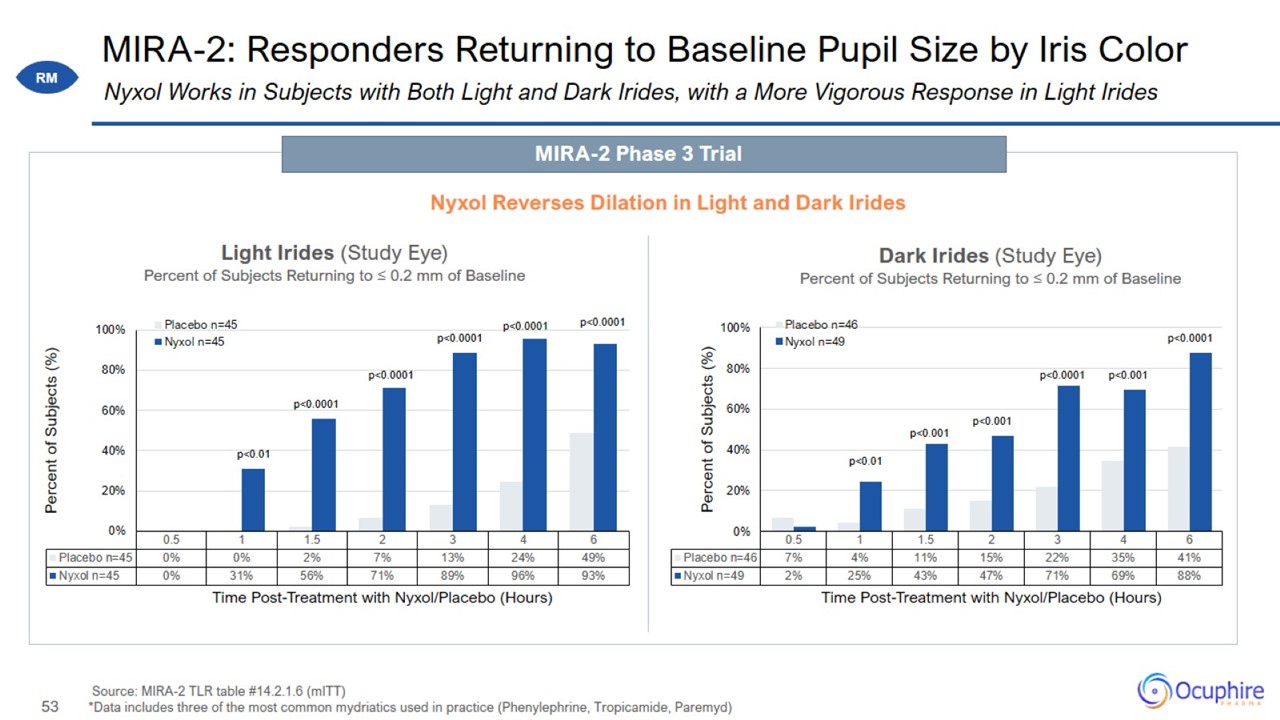
MIRA-2: Responders Returning to Baseline Pupil Size by Iris Color Source: MIRA-2 TLR table #14.2.1.6 (mITT) *Data includes three
of the most common mydriatics used in practice (Phenylephrine, Tropicamide, Paremyd) Nyxol Works in Subjects with Both Light and Dark Irides, with a More Vigorous Response in Light Irides
MIRA-2: Responders Returning to Baseline Pupil Size by Iris Color Source: MIRA-2 TLR table #14.2.1.6 (mITT) *Data includes three
of the most common mydriatics used in practice (Phenylephrine, Tropicamide, Paremyd) Nyxol Works in Subjects with Both Light and Dark Irides, with a More Vigorous Response in Light Irides
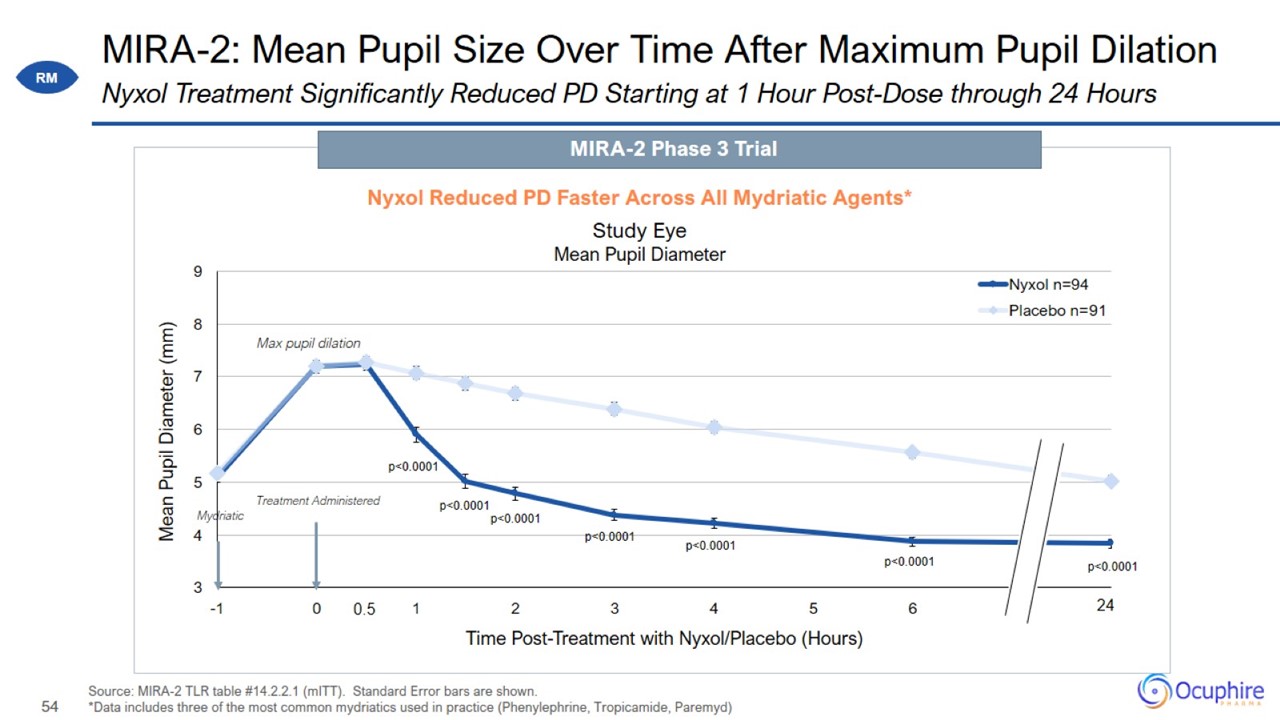
MIRA-2: Mean Pupil Size Over Time After Maximum Pupil Dilation Source: MIRA-2 TLR table #14.2.2.1 (mITT). Standard Error bars are
shown. *Data includes three of the most common mydriatics used in practice (Phenylephrine, Tropicamide, Paremyd) Nyxol Treatment Significantly Reduced PD Starting at 1 Hour Post-Dose through 24 Hours
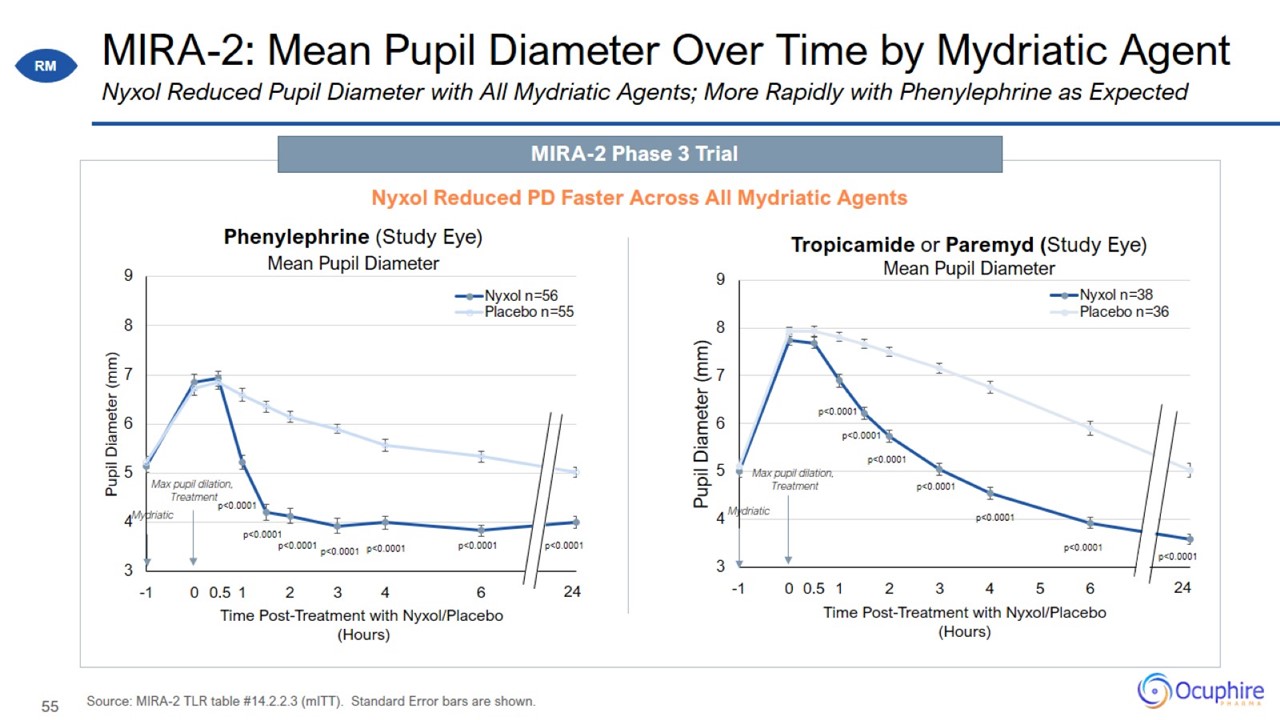
MIRA-2: Mean Pupil Diameter Over Time by Mydriatic Agent Source: MIRA-2 TLR table #14.2.2.3 (mITT). Standard Error bars are
shown. Nyxol Reduced Pupil Diameter with All Mydriatic Agents; More Rapidly with Phenylephrine as Expected
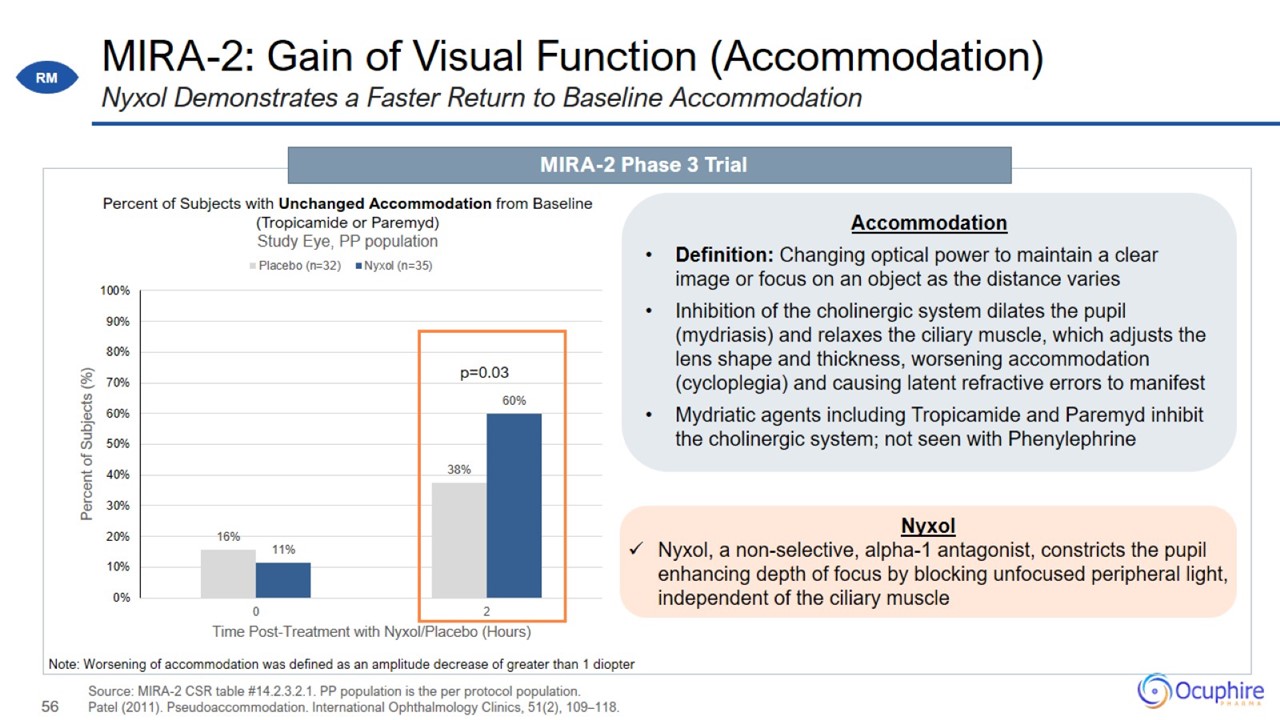
MIRA-2: Gain of Visual Function (Accommodation) Source: MIRA-2 CSR table #14.2.3.2.1. PP population is the per protocol
population. Patel (2011). Pseudoaccommodation. International Ophthalmology Clinics, 51(2), 109–118. Nyxol Demonstrates a Faster Return to Baseline Accommodation
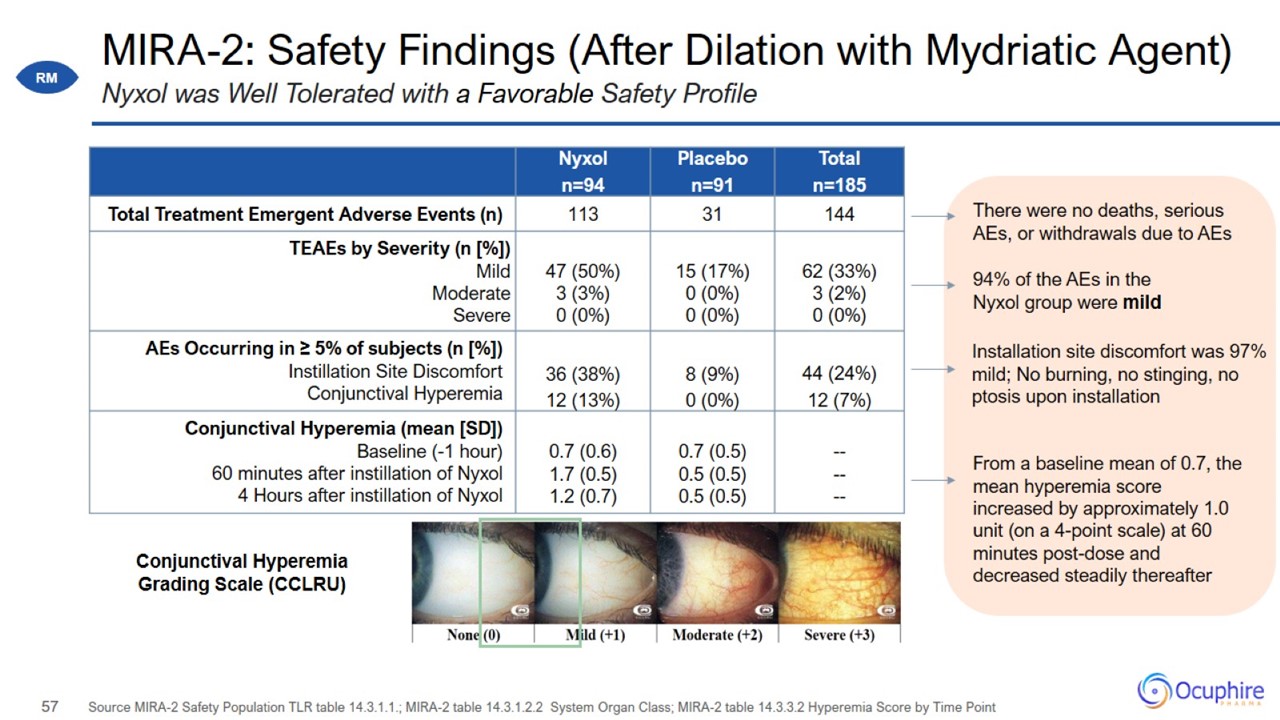
MIRA-2: Safety Findings (After Dilation with Mydriatic Agent) Source MIRA-2 Safety Population TLR table 14.3.1.1.; MIRA-2 table
14.3.1.2.2 System Organ Class; MIRA-2 table 14.3.3.2 Hyperemia Score by Time Point Nyxol was Well Tolerated with a Favorable Safety Profile
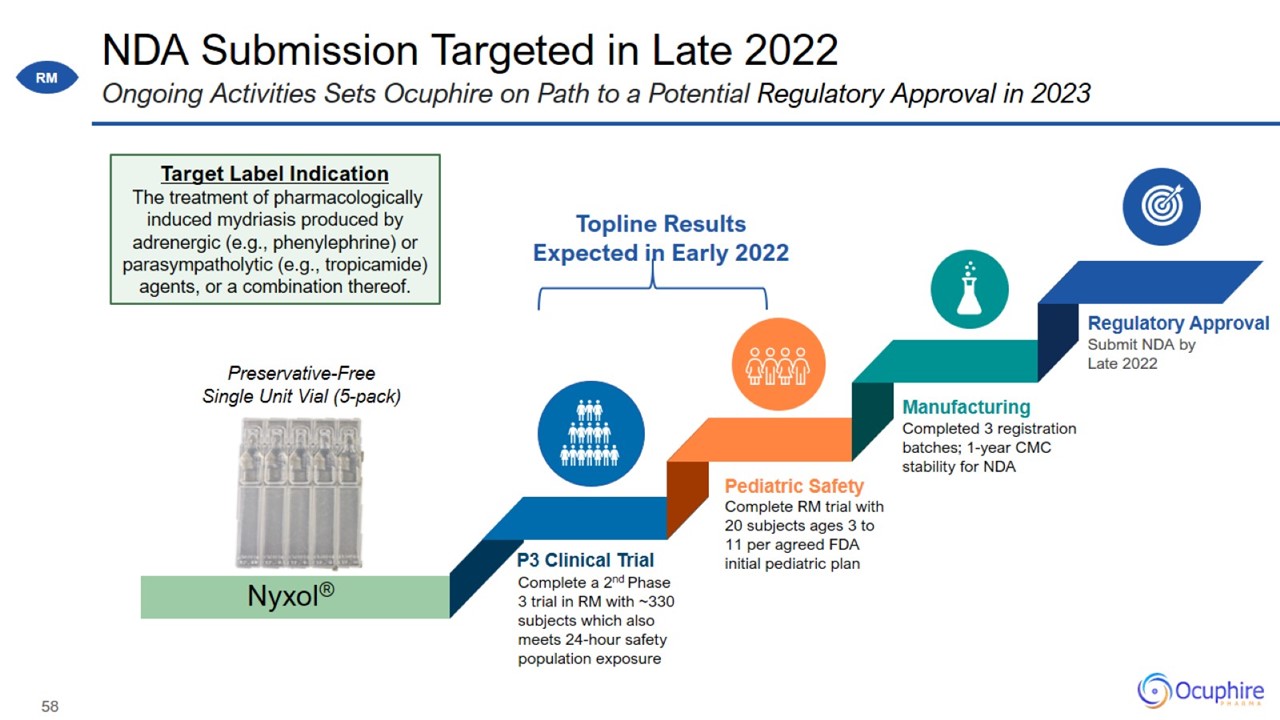
NDA Submission Targeted in Late 2022 Ongoing Activities Sets Ocuphire on Path to a Potential Regulatory Approval in 2023
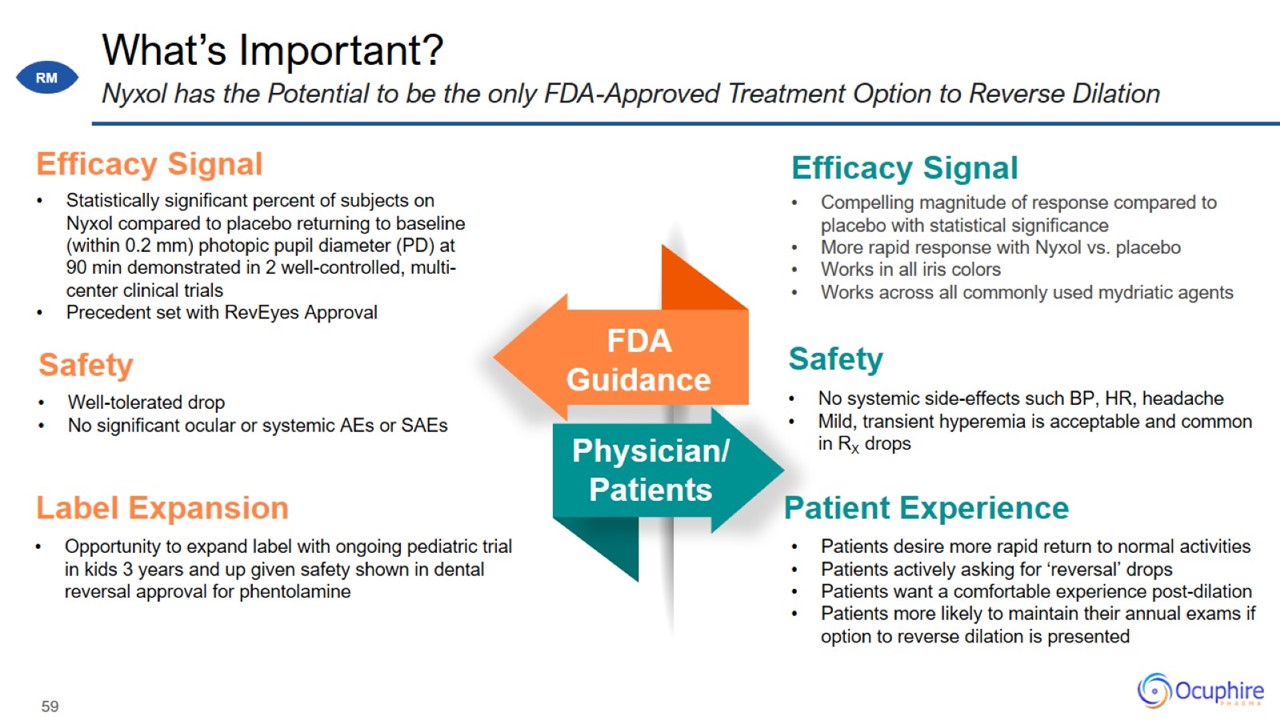
What’s Important? Nyxol has the Potential to be the only FDA-Approved Treatment Option to Reverse Dilation

RM Market Opportunity and Commercialization Patients are Vocal About the Negative Effects of Mydriasis Quotes are from anonymous
patients
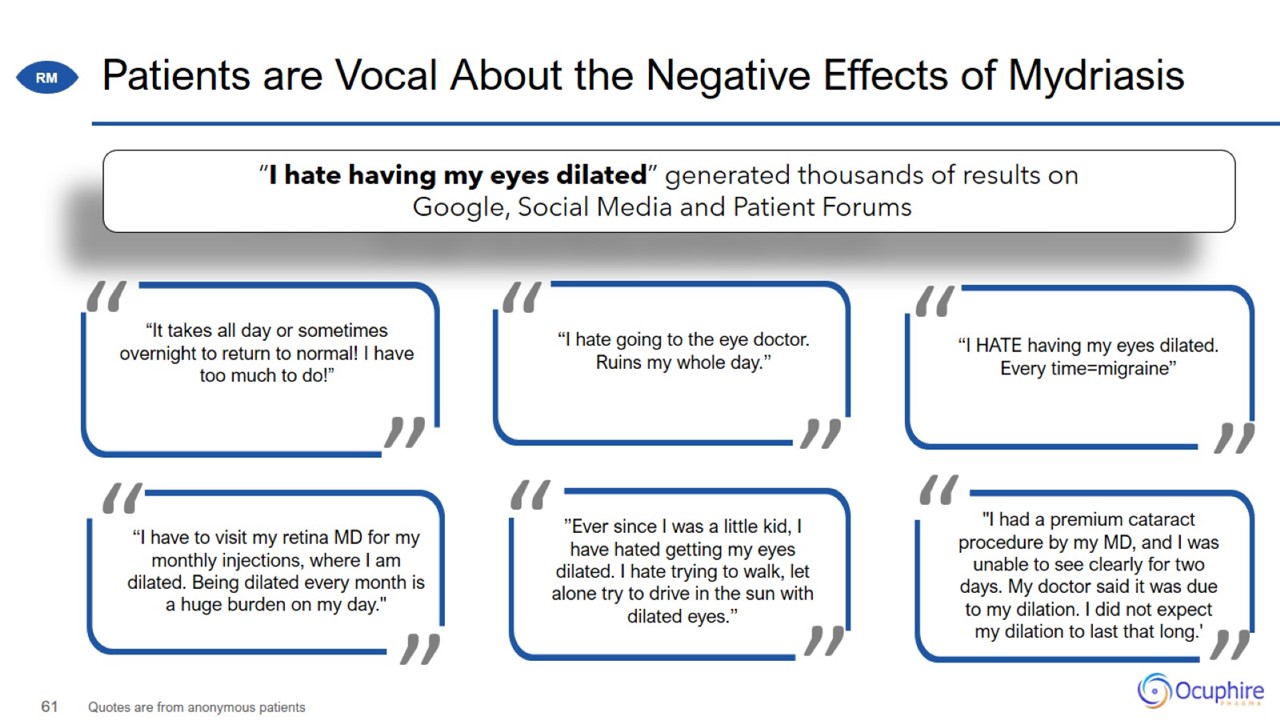
Patients are Vocal About the Negative Effects of Mydriasis Quotes are from anonymous patients
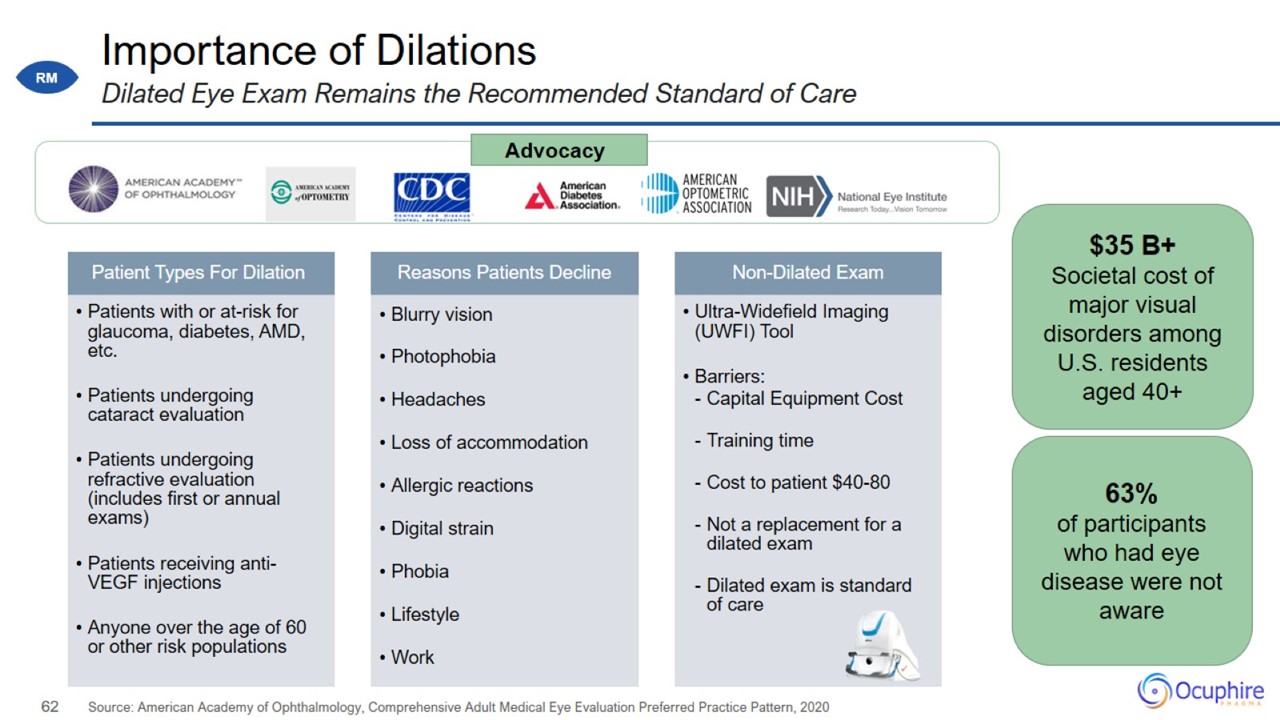
Importance of Dilations Source: American Academy of Ophthalmology, Comprehensive Adult Medical Eye Evaluation Preferred Practice
Pattern, 2020 Dilated Eye Exam Remains the Recommended Standard of Care
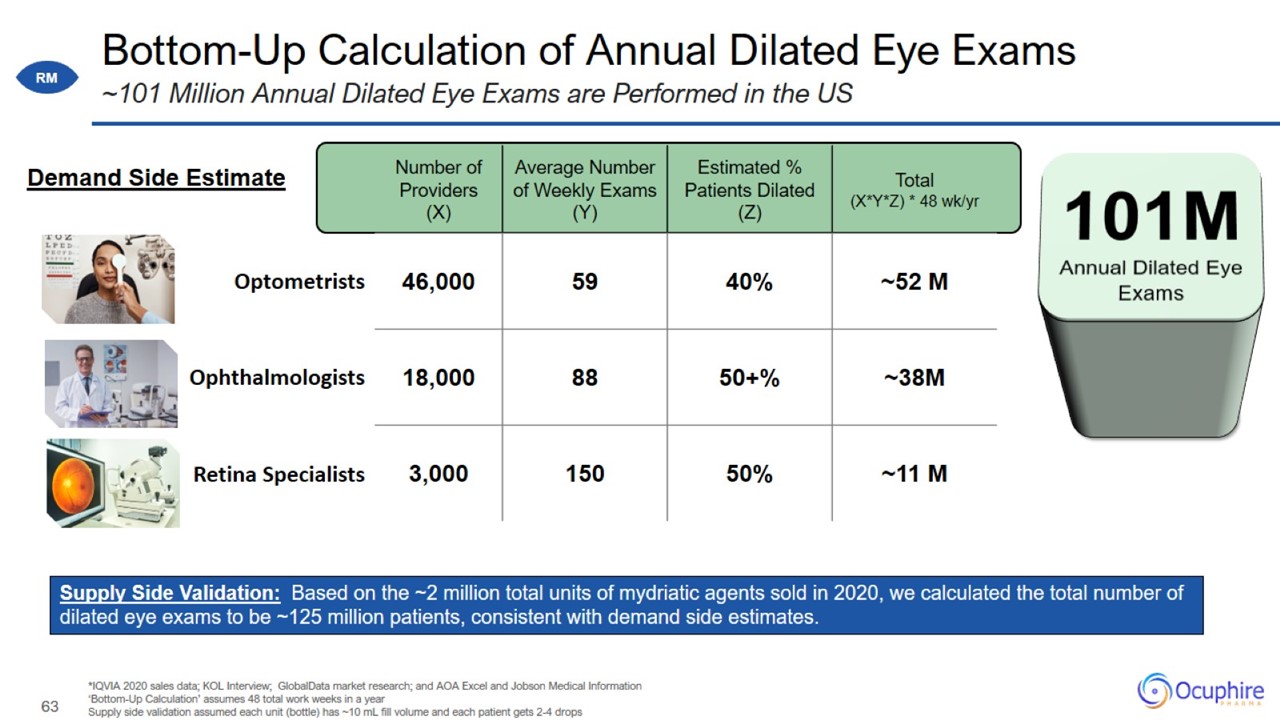
Bottom-Up Calculation of Annual Dilated Eye Exams *IQVIA 2020 sales data; KOL Interview; GlobalData market research; and AOA
Excel and Jobson Medical Information ‘Bottom-Up Calculation’ assumes 48 total work weeks in a year Supply side validation assumed each unit (bottle) has ~10 mL fill volume and each patient gets 2-4 drops ~101 Million Annual Dilated Eye Exams are
Performed in the US
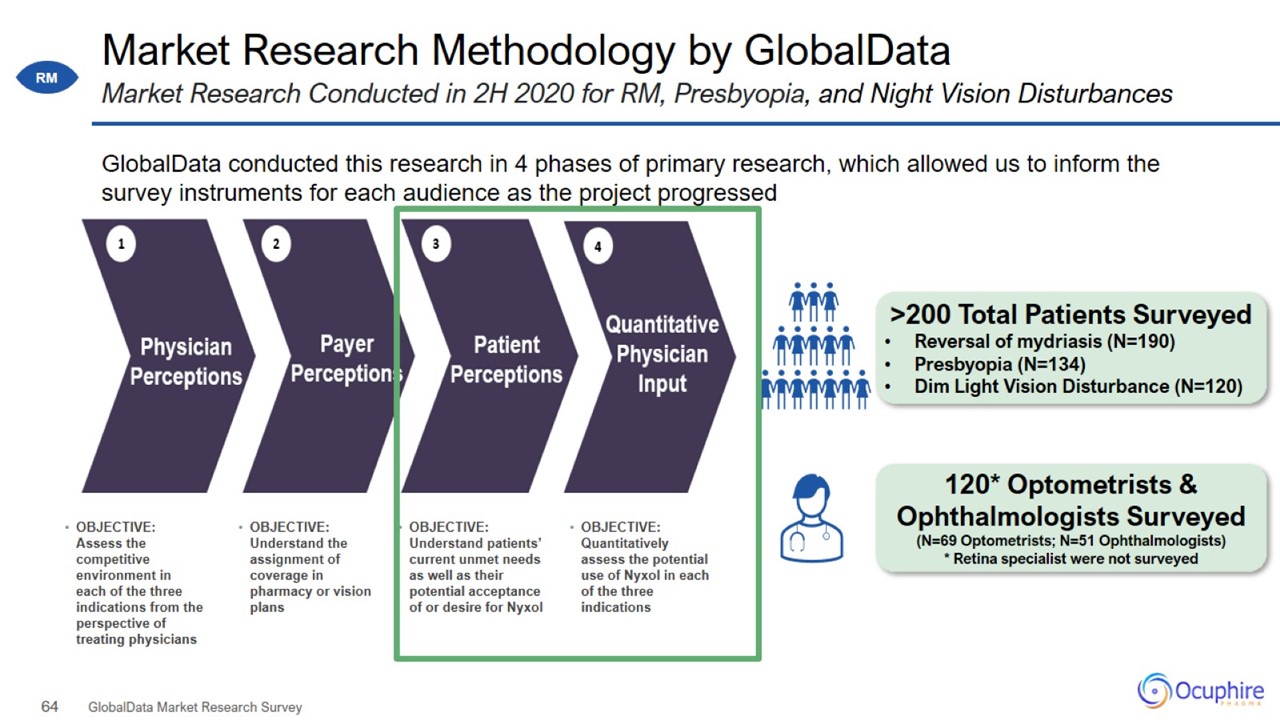
Market Research Methodology by GlobalData GlobalData Market Research Survey Market Research Conducted in 2H 2020 for RM,
Presbyopia, and Night Vision Disturbances
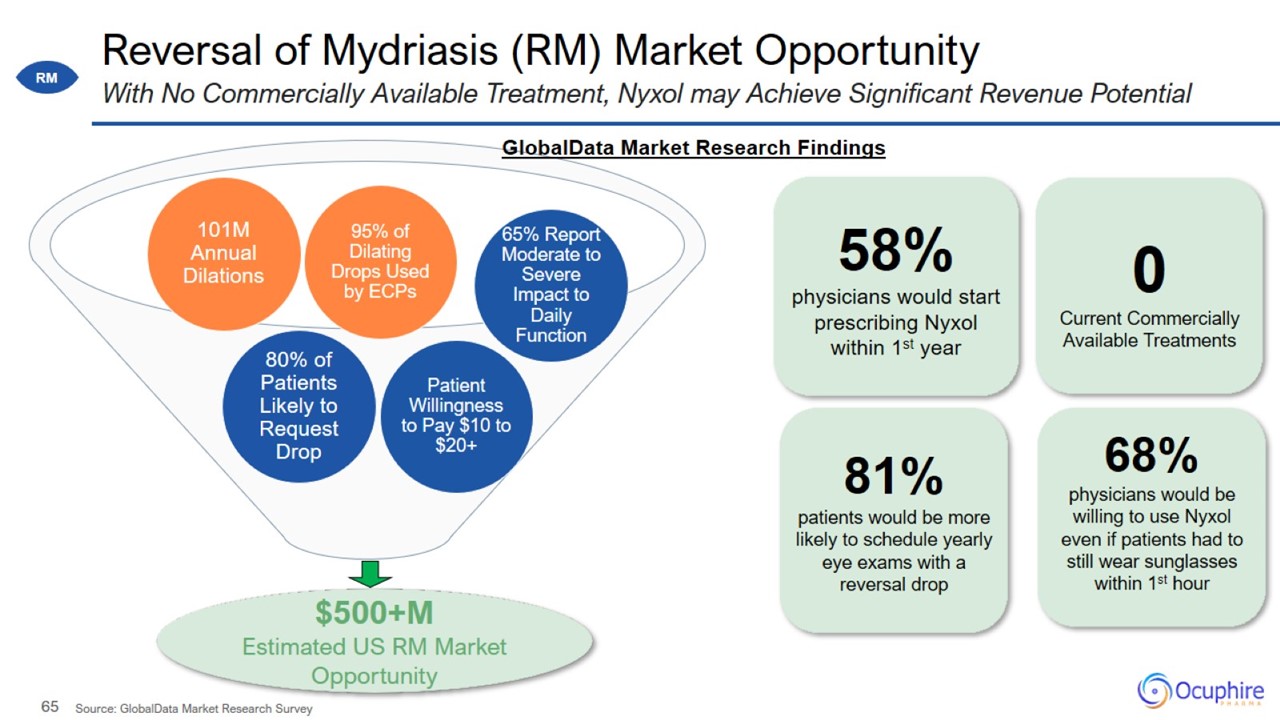
Reversal of Mydriasis (RM) Market Opportunity With No Commercially Available Treatment, Nyxol may Achieve Significant Revenue
Potential
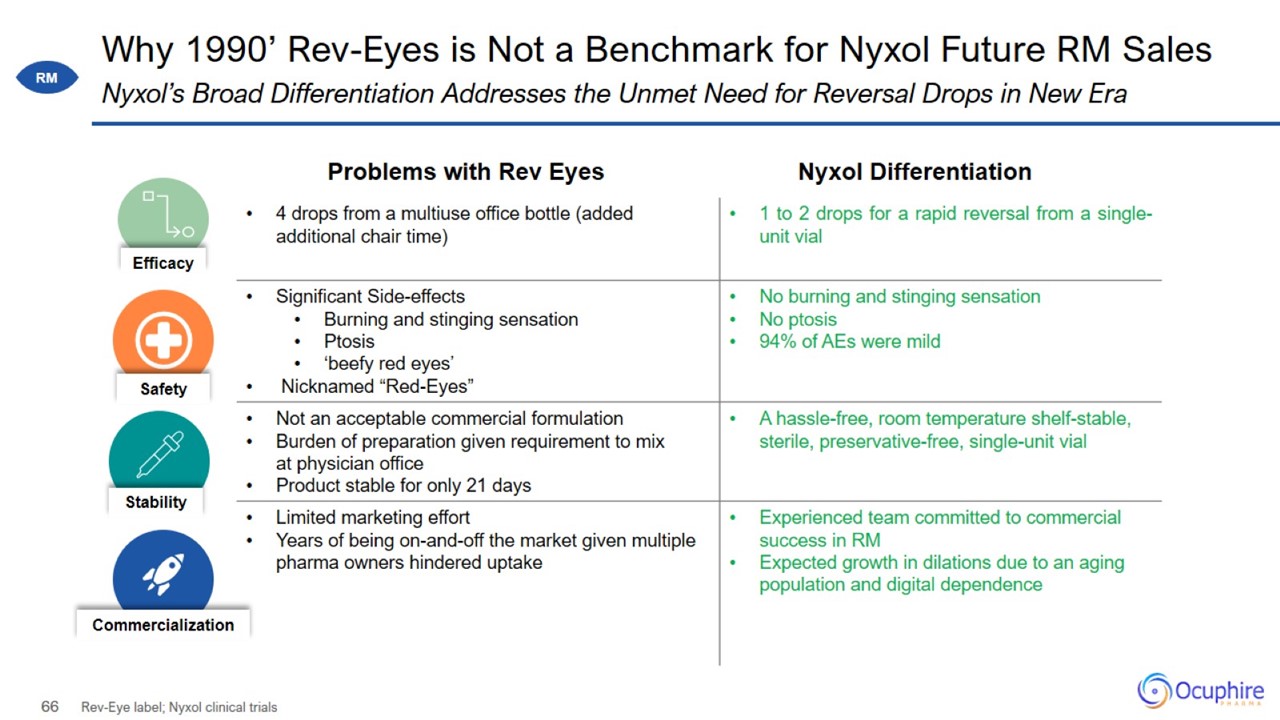
Why 1990’ Rev-Eyes is Not a Benchmark for Nyxol Future RM Sales Rev-Eye label; Nyxol clinical trials Nyxol’s Broad
Differentiation Addresses the Unmet Need for Reversal Drops in New Era

RM Market Opportunity and Commercialization

Perspective from Practice Administrators on Reversing Dilations Anterior Segment Practice in Southeast “We’ve explored and
offered several options over the years to reverse mydriasis – both as a tool to elevate the patient experience and to reduce liability when a patient with poor accommodation walks out of my practice. We’ve used pharmacologic agents to reverse the
effects of dilation in the past, but those products had significant limitations, and I would welcome a new option indicated for RM in my practice.” – Certified Ophthalmic Executive and CEO Multi-Specialty Practice, Midwest “We call our
patients guests so anything to enhance their experience is valuable for our practice. Pupil dilation is a perceived inconvenience – especially for the working-age population. They grab up our evening appointments typically, so they don’t have to
go back to work while dilated. Having an option to reverse dilation is something we and our guests would enjoy." – Certified Ophthalmic Executive and CEO Leading Academic Eye Center “Patients have anxiety over dilation and if we could reduce
that fear, help them regain accommodation faster, I would like to consider that option across all specialties." – Head of Operations, Certified Practice Administrator Interviews Conducted by Ocuphire Leading Practice Administrators Confirm the
‘Market Need,’ and Value to Patient

Pre-Commercial Activities in 2022 Activities Underway to Support Capital-Efficient Nyxol RM Commercial Launch
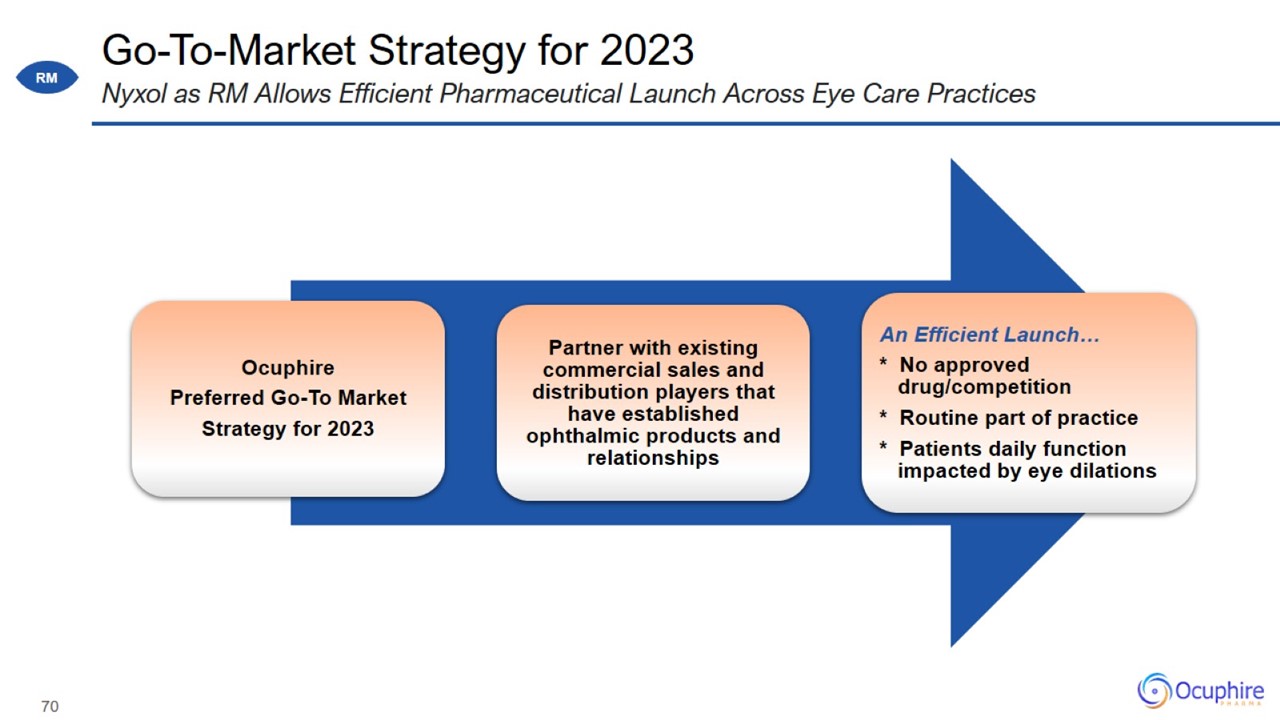
Go-To-Market Strategy for 2023 Nyxol as RM Allows Efficient Pharmaceutical Launch Across Eye Care Practices
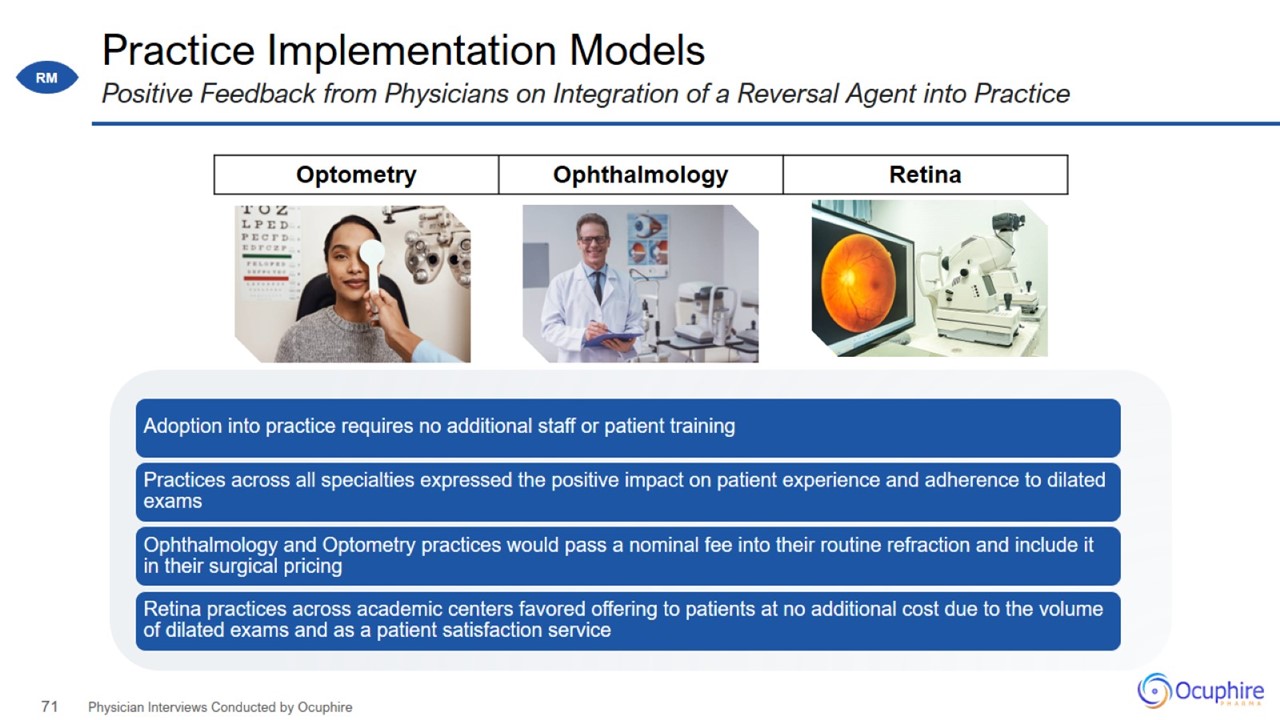
Practice Implementation Models Physician Interviews Conducted by Ocuphire Positive Feedback from Physicians on Integration of a
Reversal Agent into Practice
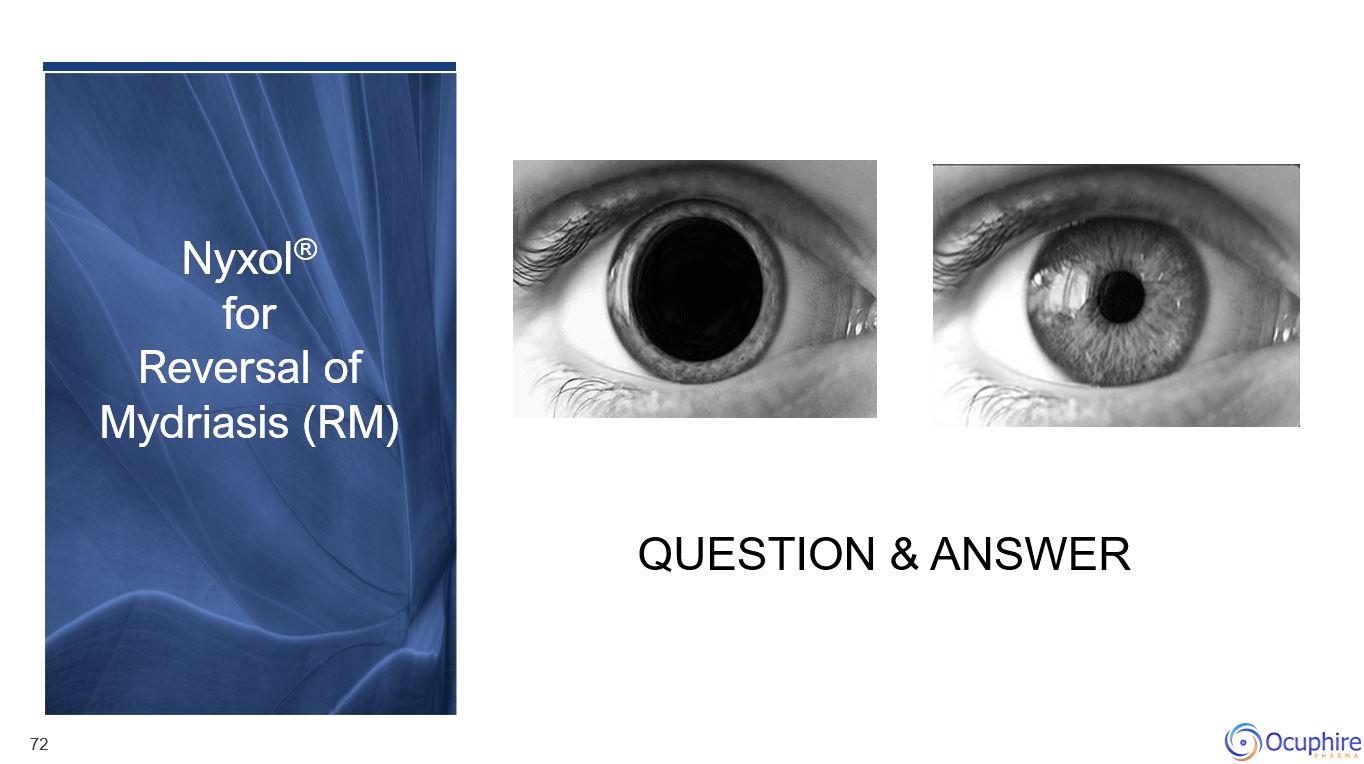
Nyxol® for Reversal of Mydriasis (RM)
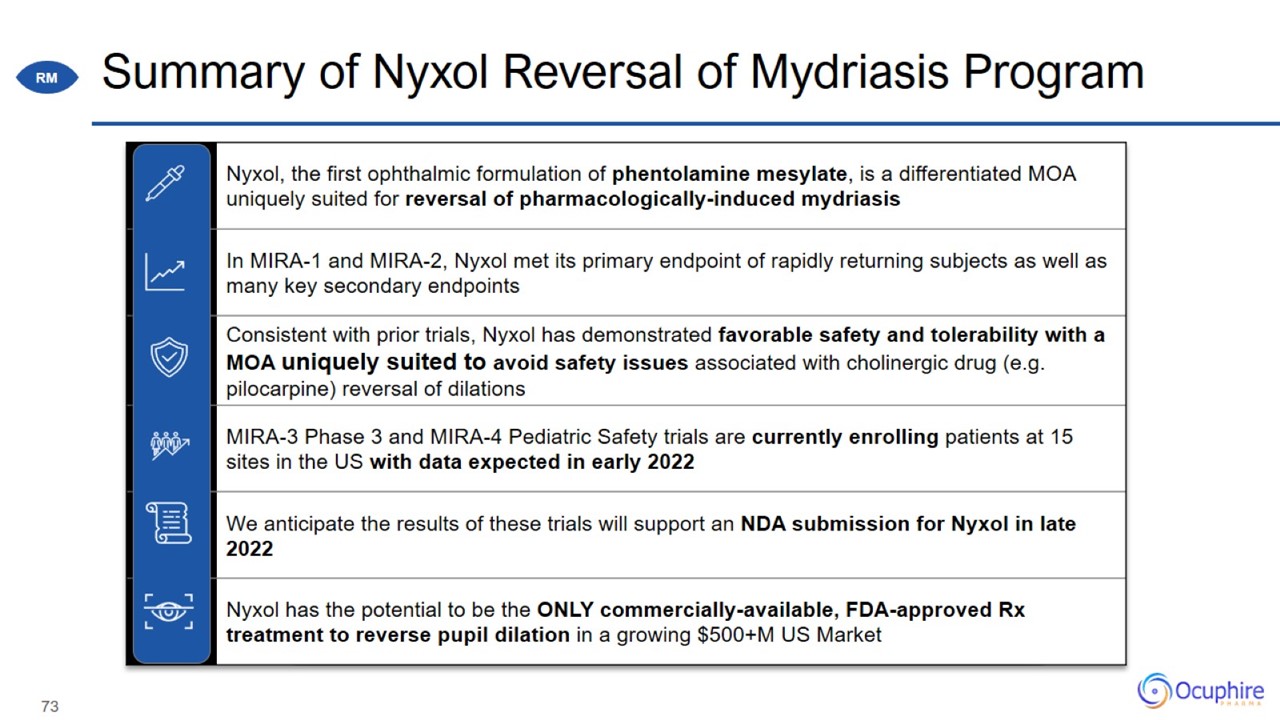
Summary of Nyxol Reversal of Mydriasis Program

III. Nyxol Presbyopia Program

Pupil Modulation Eye Drops for Presbyopia
The Time for Presbyopia Drops Sources: Academic review articles, journals, and publications Headlines from Academia and Industry
Articles with an Early First Approval for VuityTM
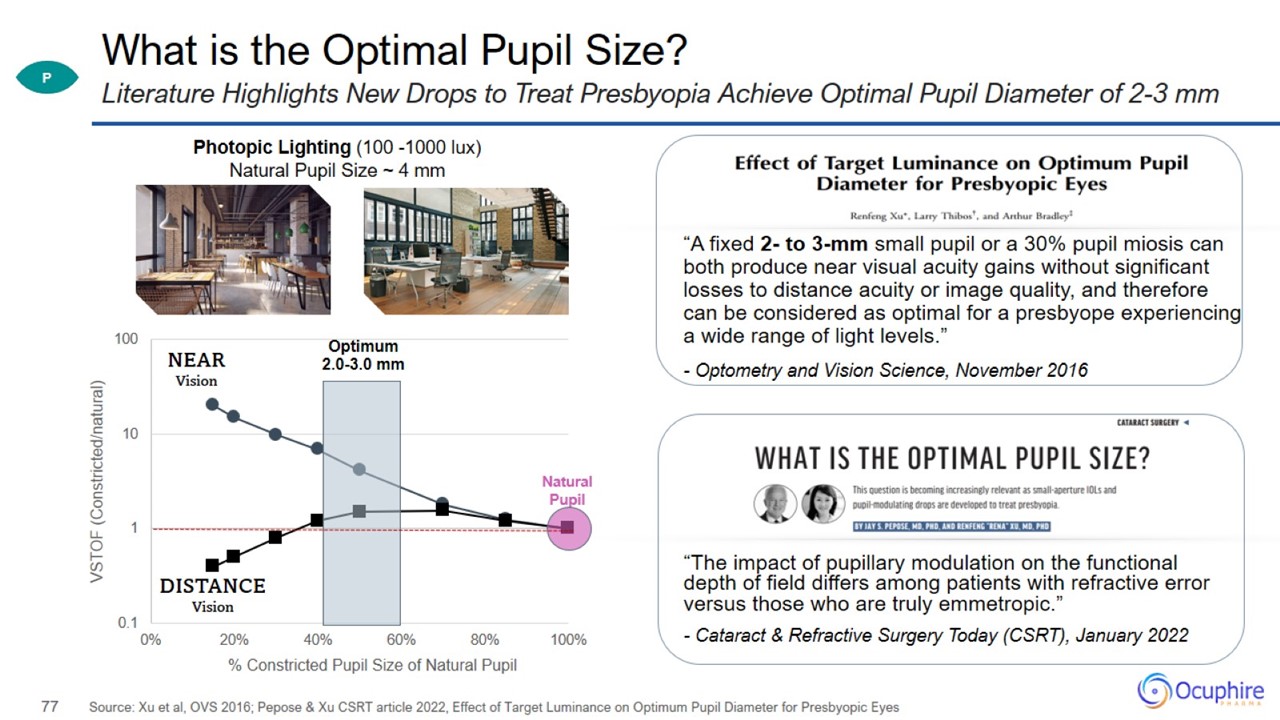
What is the Optimal Pupil Size? “A fixed 2- to 3-mm small pupil or a 30% pupil miosis can both produce near visual acuity gains
without significant losses to distance acuity or image quality, and therefore can be considered as optimal for a presbyope experiencing a wide range of light levels.” - Optometry and Vision Science, November 2016 Source: Xu et al, OVS 2016;
Pepose & Xu CSRT article 2022, Effect of Target Luminance on Optimum Pupil Diameter for Presbyopic Eyes Literature Highlights New Drops to Treat Presbyopia Achieve Optimal Pupil Diameter of 2-3 mm

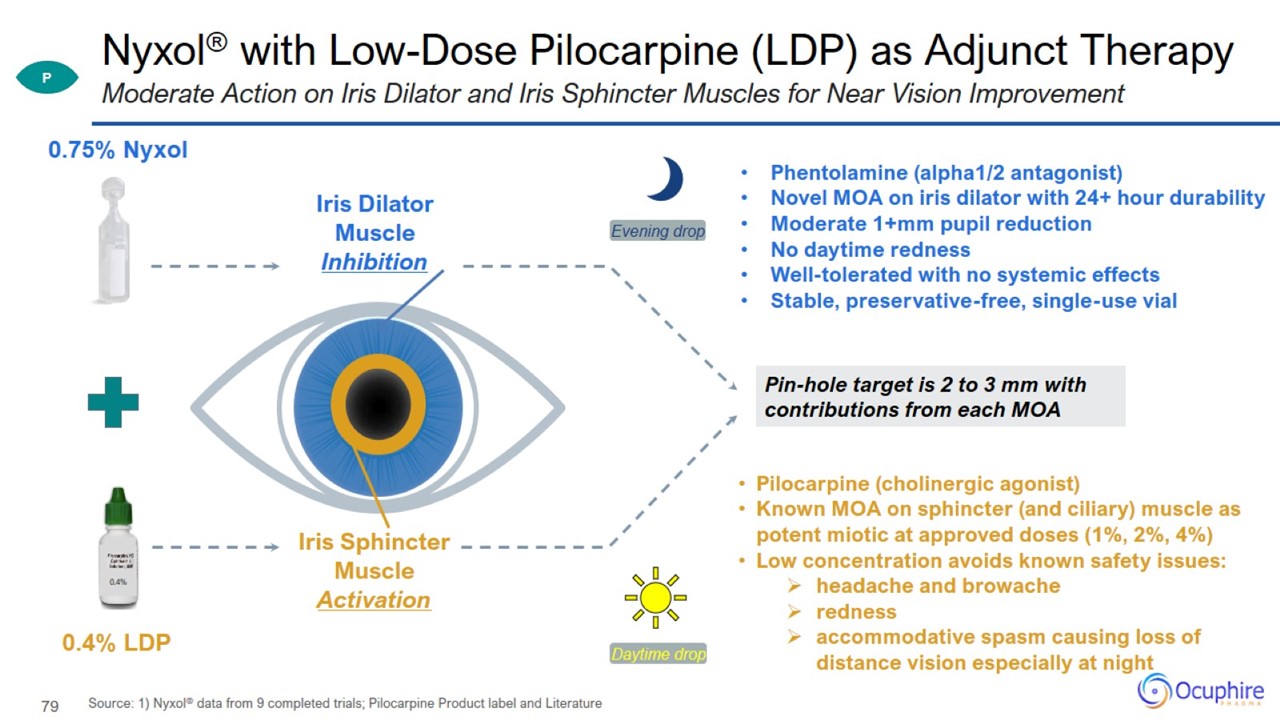
Nyxol® with Low-Dose Pilocarpine (LDP) as Adjunct Therapy Source: 1) Nyxol® data from 9 completed trials; Pilocarpine Product
label and Literature Moderate Action on Iris Dilator and Iris Sphincter Muscles for Near Vision Improvement
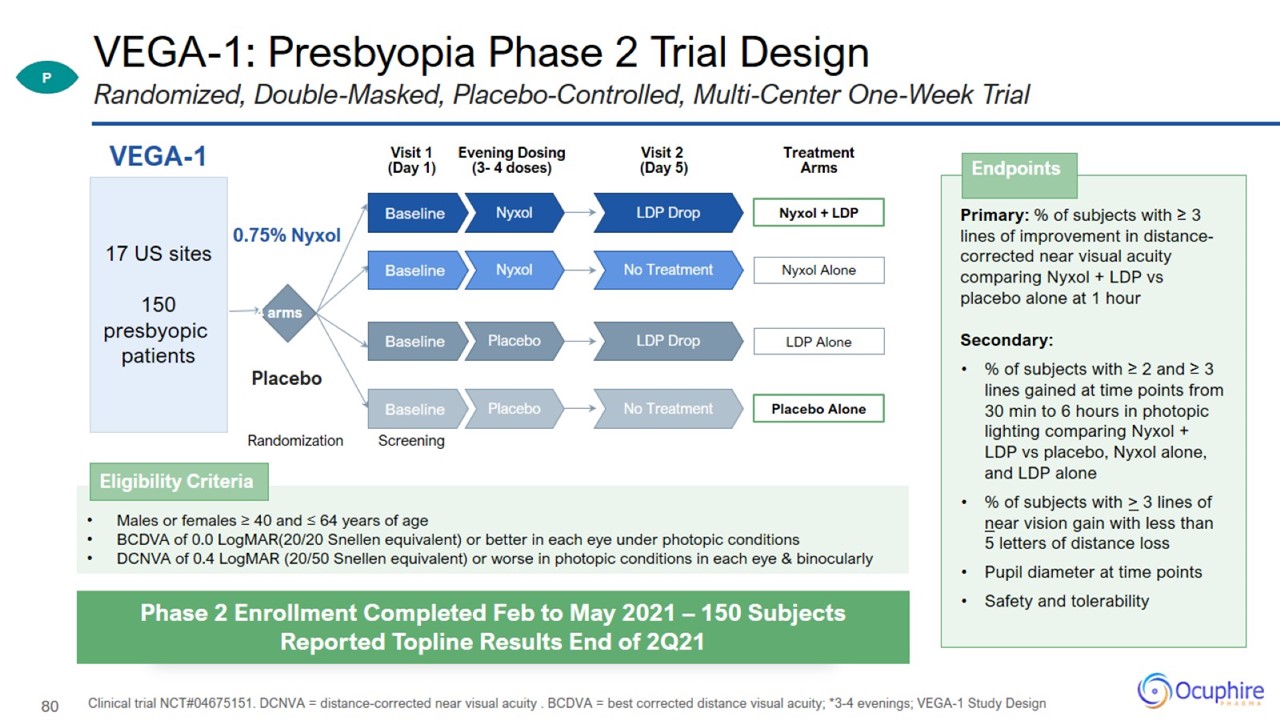
VEGA-1: Presbyopia Phase 2 Trial Design Clinical trial NCT#04675151. DCNVA = distance-corrected near visual acuity . BCDVA = best
corrected distance visual acuity; *3-4 evenings; VEGA-1 Study Design Randomized, Double-Masked, Placebo-Controlled, Multi-Center One-Week Trial
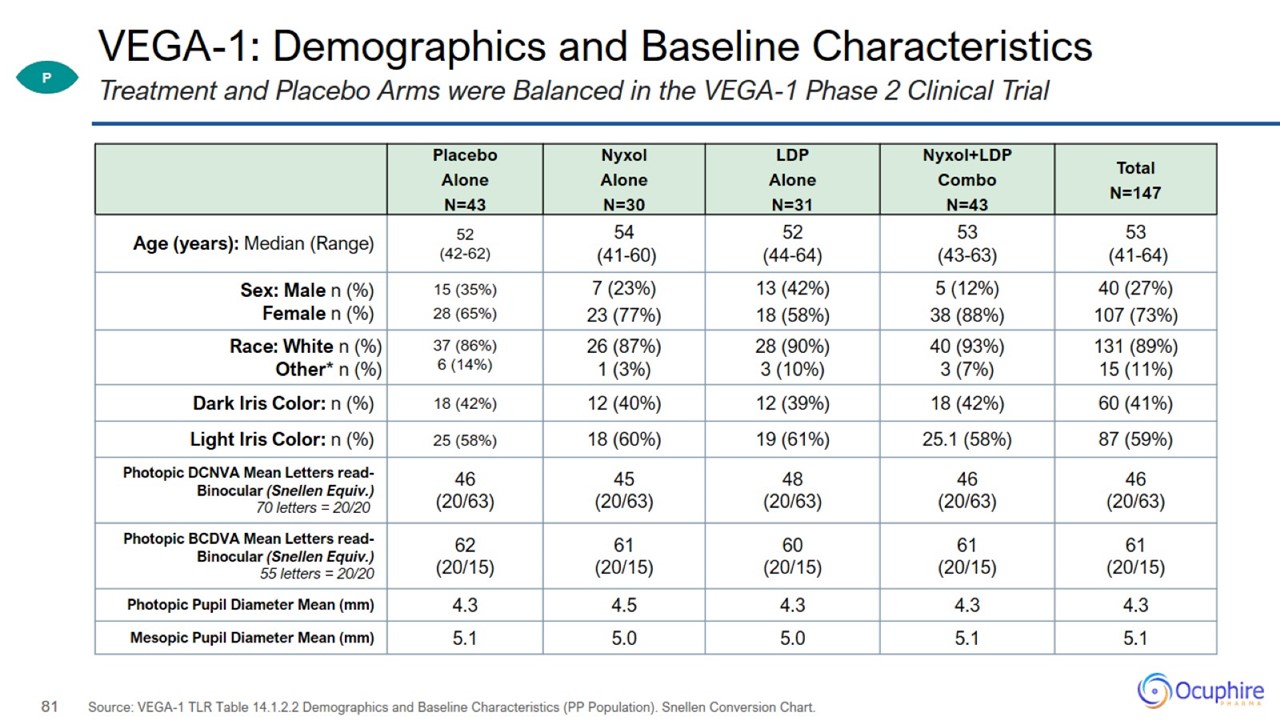
VEGA-1: Demographics and Baseline Characteristics Source: VEGA-1 TLR Table 14.1.2.2 Demographics and Baseline Characteristics (PP
Population). Snellen Conversion Chart. Treatment and Placebo Arms were Balanced in the VEGA-1 Phase 2 Clinical Trial
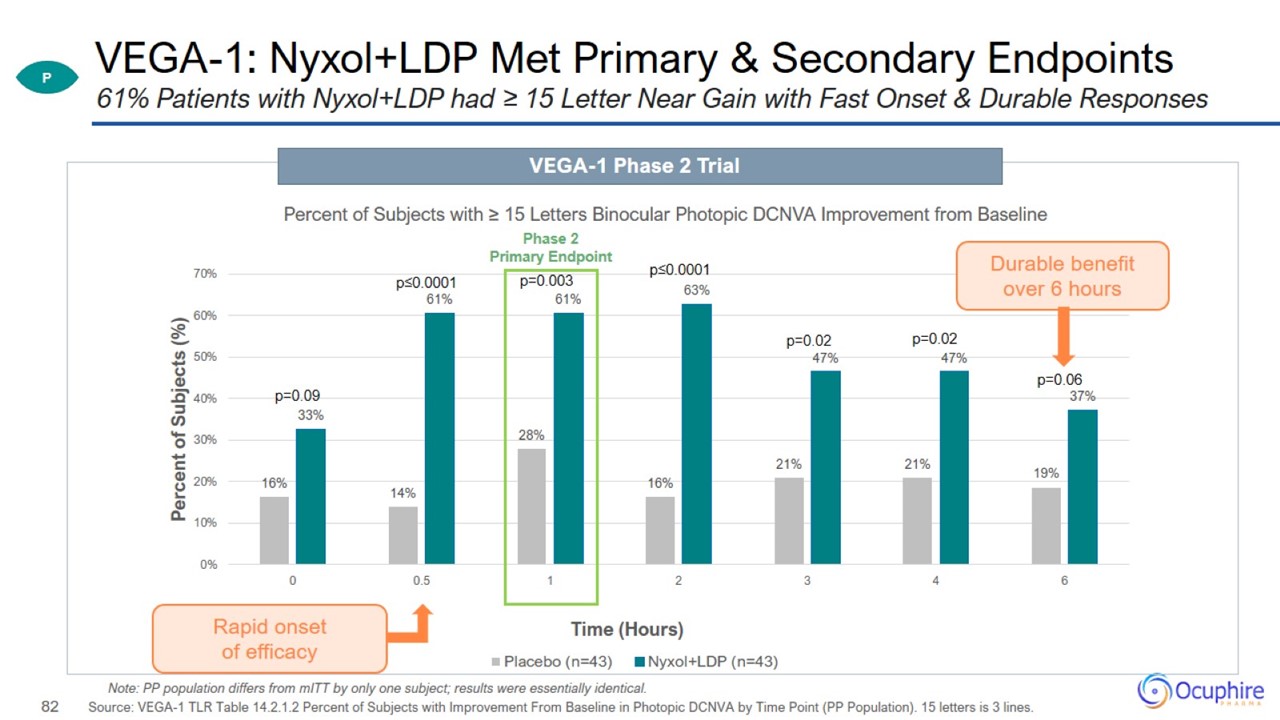
VEGA-1: Nyxol+LDP Met Primary & Secondary Endpoints Source: VEGA-1 TLR Table 14.2.1.2 Percent of Subjects with Improvement
From Baseline in Photopic DCNVA by Time Point (PP Population). 15 letters is 3 lines. 61% Patients with Nyxol+LDP had ≥ 15 Letter Near Gain with Fast Onset & Durable Responses
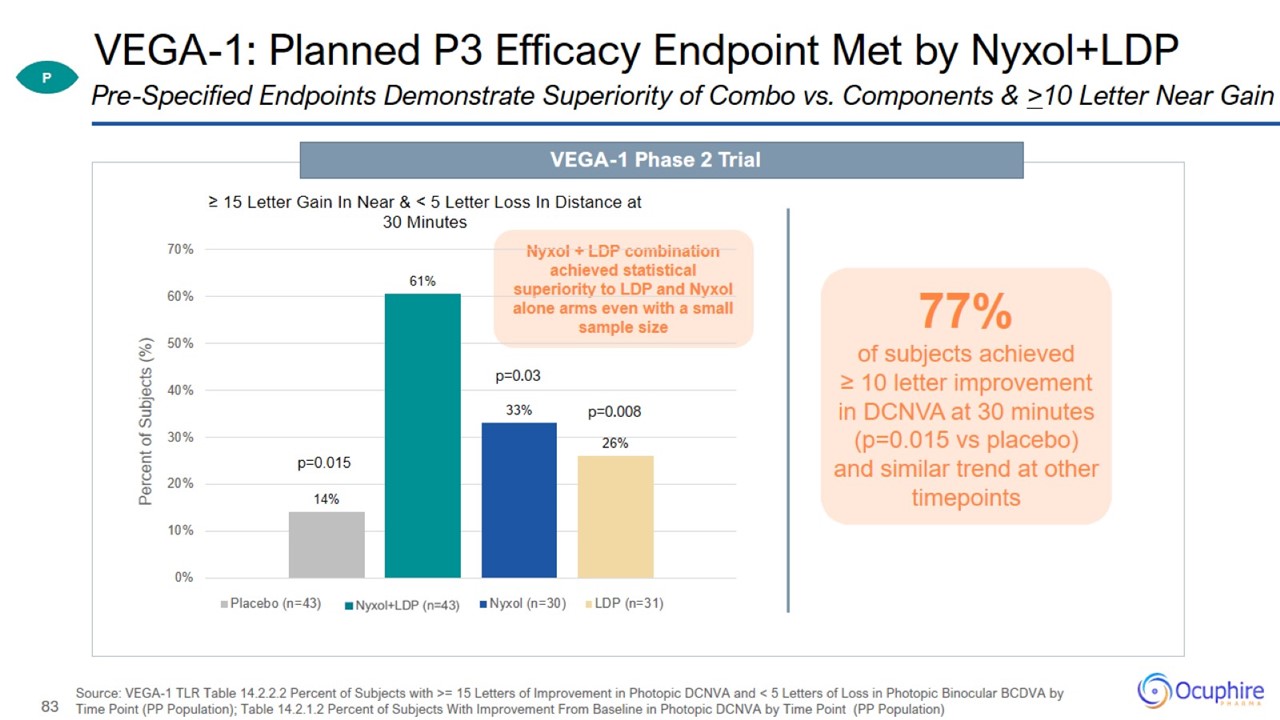
VEGA-1: Planned P3 Efficacy Endpoint Met by Nyxol+LDP Source: VEGA-1 TLR Table 14.2.2.2 Percent of Subjects with >= 15 Letters
of Improvement in Photopic DCNVA and < 5 Letters of Loss in Photopic Binocular BCDVA by Time Point (PP Population); Table 14.2.1.2 Percent of Subjects With Improvement From Baseline in Photopic DCNVA by Time Point (PP Population)
Pre-Specified Endpoints Demonstrate Superiority of Combo vs. Components & >10 Letter Near Gain
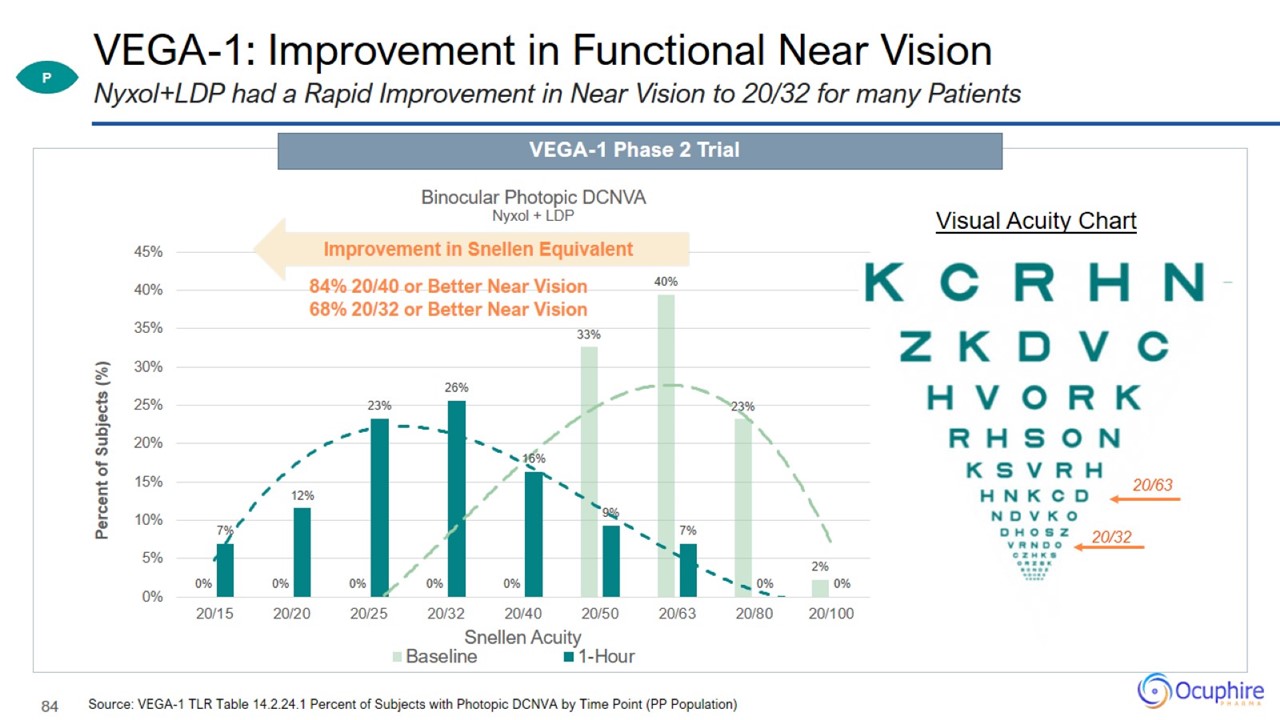
VEGA-1: Improvement in Functional Near Vision Source: VEGA-1 TLR Table 14.2.24.1 Percent of Subjects with Photopic DCNVA by Time
Point (PP Population) Nyxol+LDP had a Rapid Improvement in Near Vision to 20/32 for many Patients
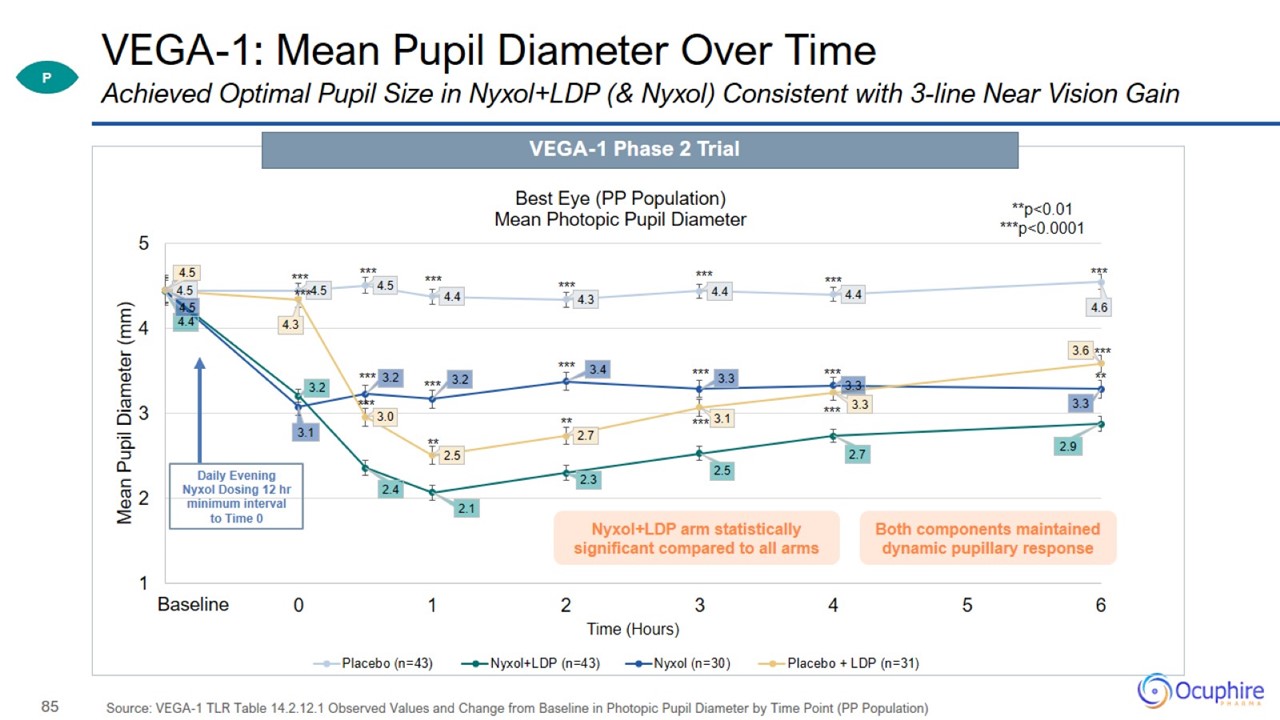
VEGA-1: Mean Pupil Diameter Over Time Source: VEGA-1 TLR Table 14.2.12.1 Observed Values and Change from Baseline in Photopic
Pupil Diameter by Time Point (PP Population) Achieved Optimal Pupil Size in Nyxol+LDP (& Nyxol) Consistent with 3-line Near Vision Gain
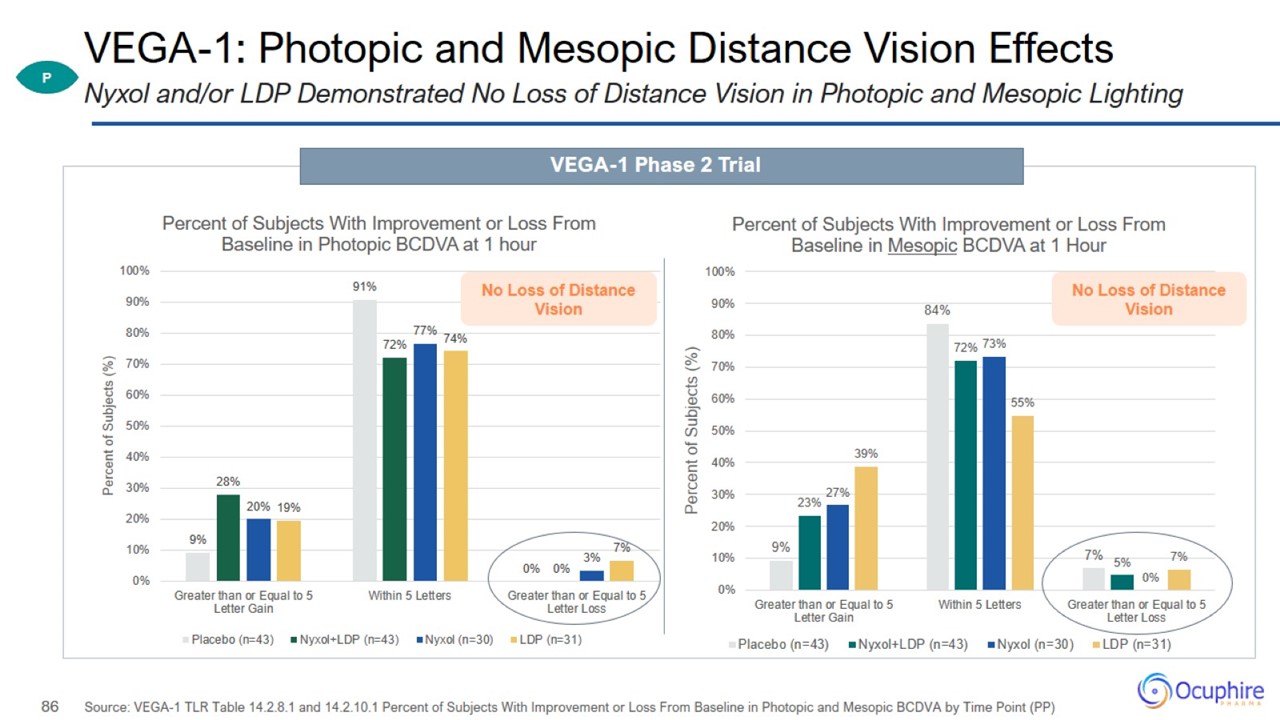
VEGA-1: Photopic and Mesopic Distance Vision Effects Source: VEGA-1 TLR Table 14.2.8.1 and 14.2.10.1 Percent of Subjects With
Improvement or Loss From Baseline in Photopic and Mesopic BCDVA by Time Point (PP) Nyxol and/or LDP Demonstrated No Loss of Distance Vision in Photopic and Mesopic Lighting
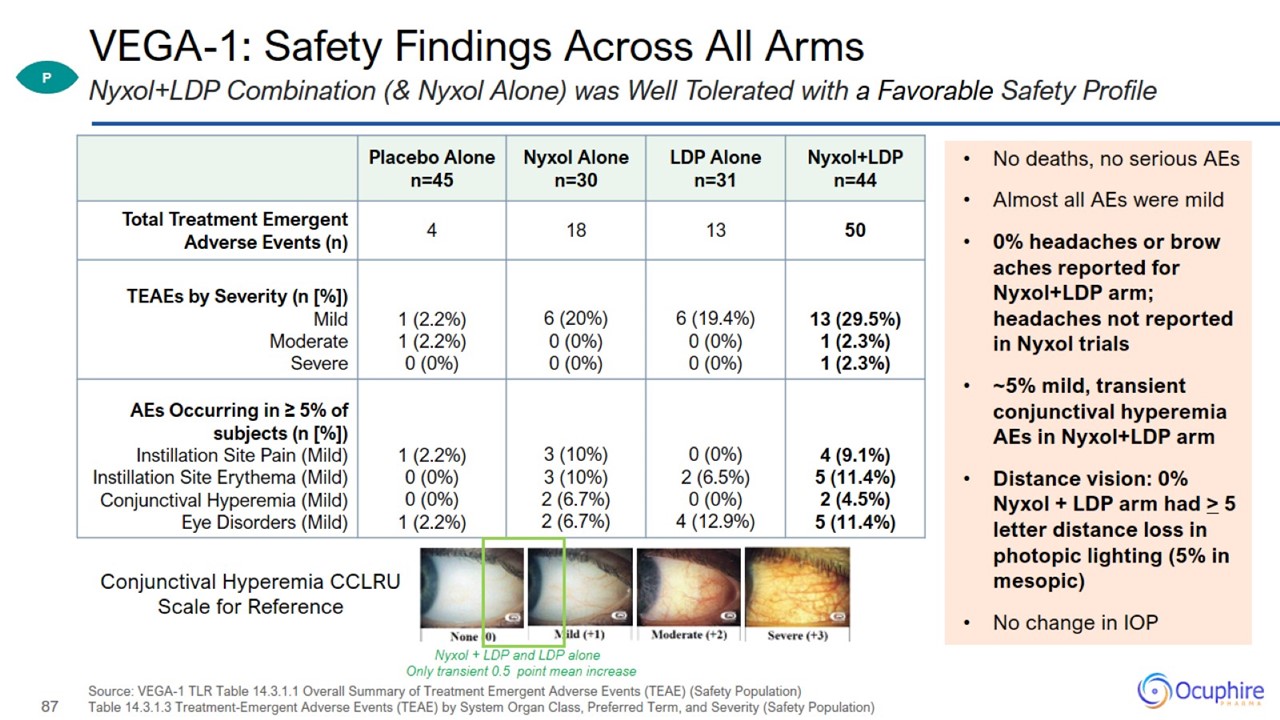
VEGA-1: Safety Findings Across All Arms Source: VEGA-1 TLR Table 14.3.1.1 Overall Summary of Treatment Emergent Adverse Events
(TEAE) (Safety Population) Table 14.3.1.3 Treatment-Emergent Adverse Events (TEAE) by System Organ Class, Preferred Term, and Severity (Safety Population) Nyxol+LDP Combination (& Nyxol Alone) was Well Tolerated with a Favorable Safety
Profile
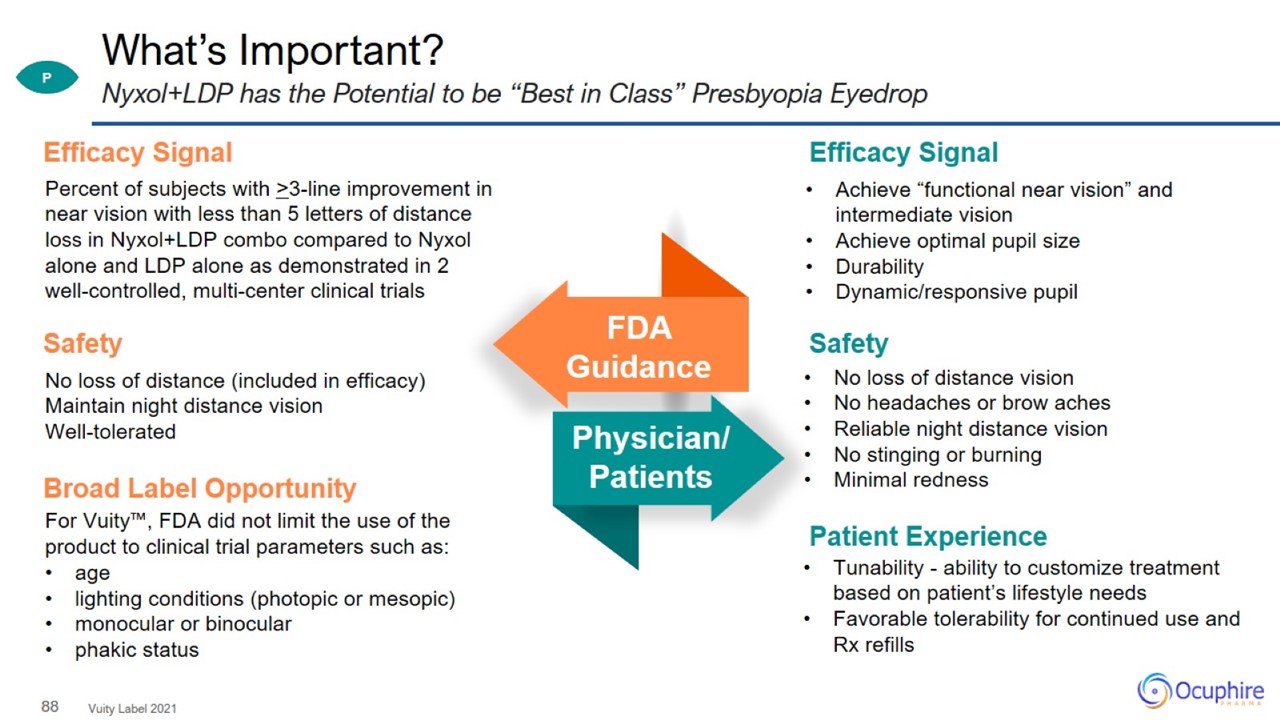
What’s Important? Vuity Label 2021 Nyxol+LDP has the Potential to be “Best in Class” Presbyopia Eyedrop

Nyxol Alone in Presbyopia
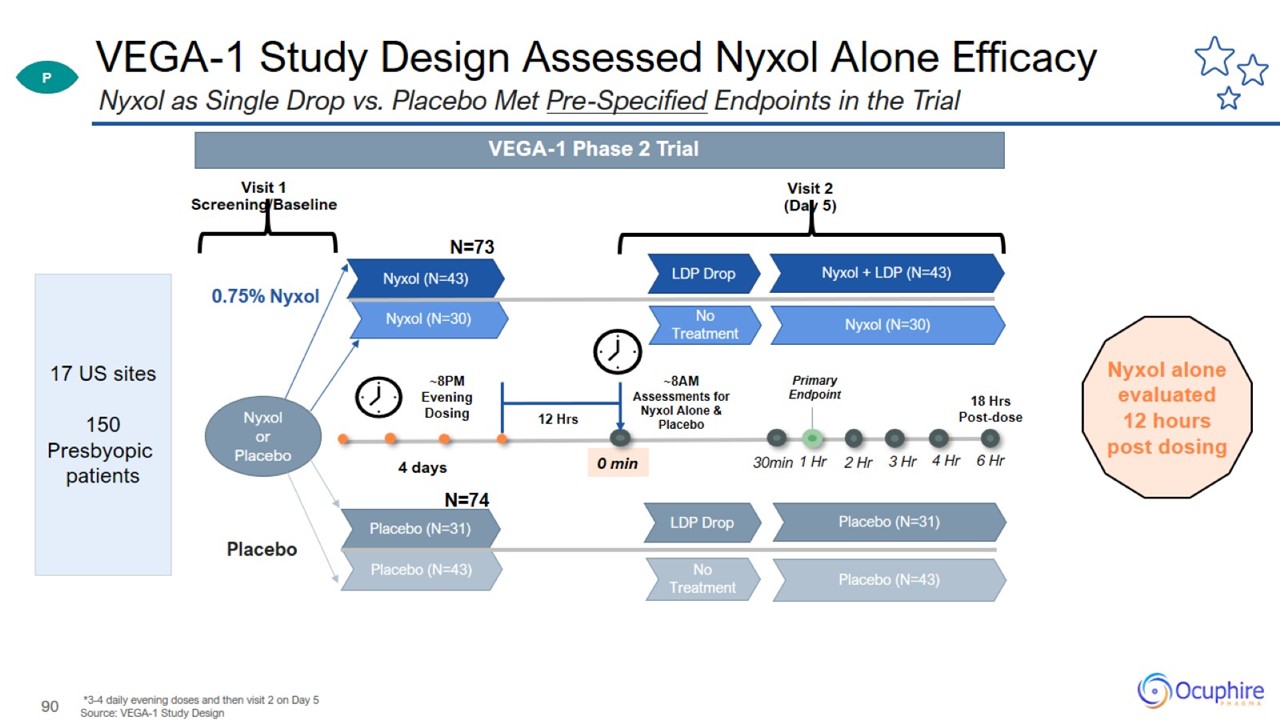
VEGA-1 Study Design Assessed Nyxol Alone Efficacy *3-4 daily evening doses and then visit 2 on Day 5 Source: VEGA-1 Study Design
Nyxol as Single Drop vs. Placebo Met Pre-Specified Endpoints in the Trial
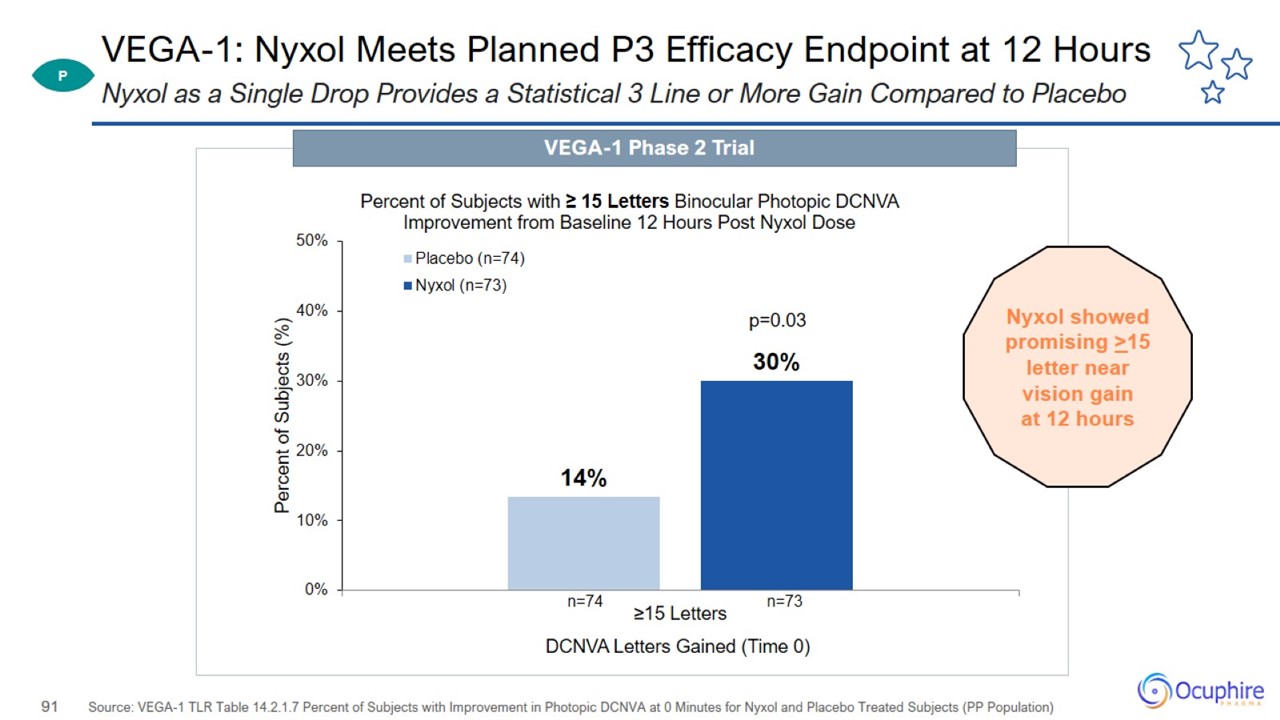
VEGA-1: Nyxol Meets Planned P3 Efficacy Endpoint at 12 Hours Source: VEGA-1 TLR Table 14.2.1.7 Percent of Subjects with
Improvement in Photopic DCNVA at 0 Minutes for Nyxol and Placebo Treated Subjects (PP Population) Nyxol as a Single Drop Provides a Statistical 3 Line or More Gain Compared to Placebo
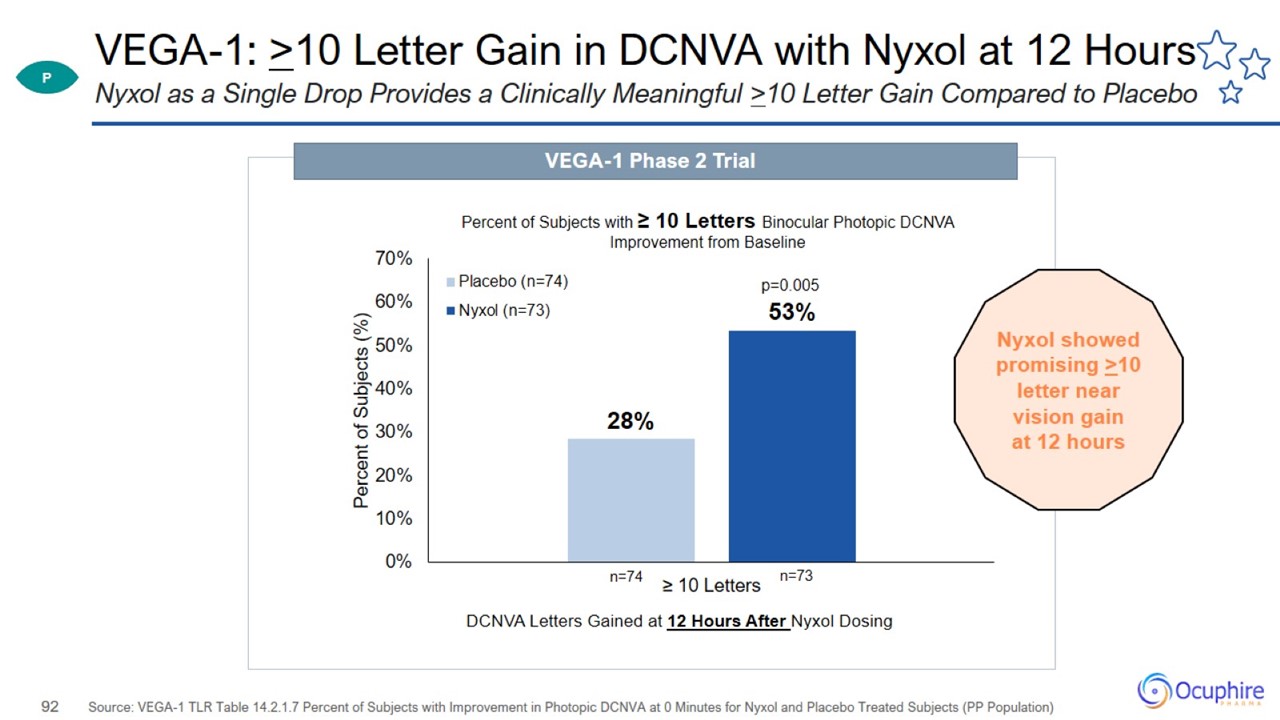
VEGA-1: >10 Letter Gain in DCNVA with Nyxol at 12 Hours Source: VEGA-1 TLR Table 14.2.1.7 Percent of Subjects with Improvement
in Photopic DCNVA at 0 Minutes for Nyxol and Placebo Treated Subjects (PP Population) Nyxol as a Single Drop Provides a Clinically Meaningful >10 Letter Gain Compared to Placebo
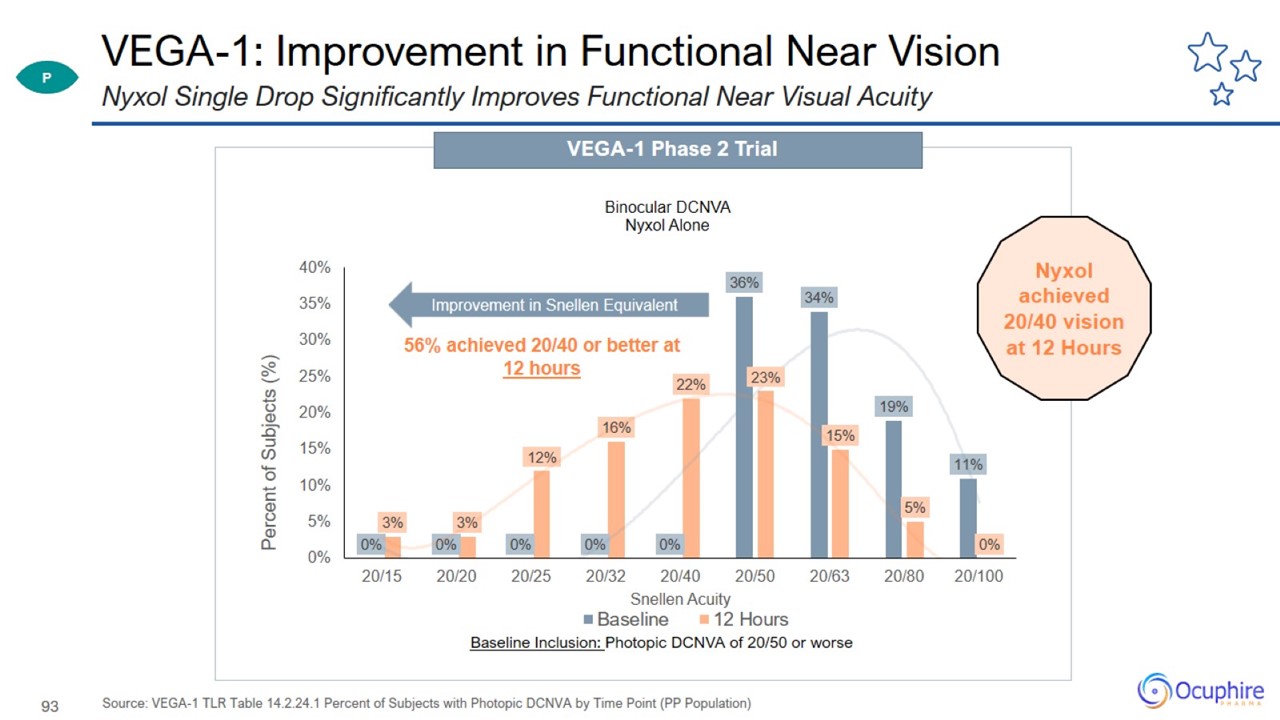
VEGA-1: Improvement in Functional Near Vision Source: VEGA-1 TLR Table 14.2.24.1 Percent of Subjects with Photopic DCNVA by Time
Point (PP Population) Nyxol Single Drop Significantly Improves Functional Near Visual Acuity
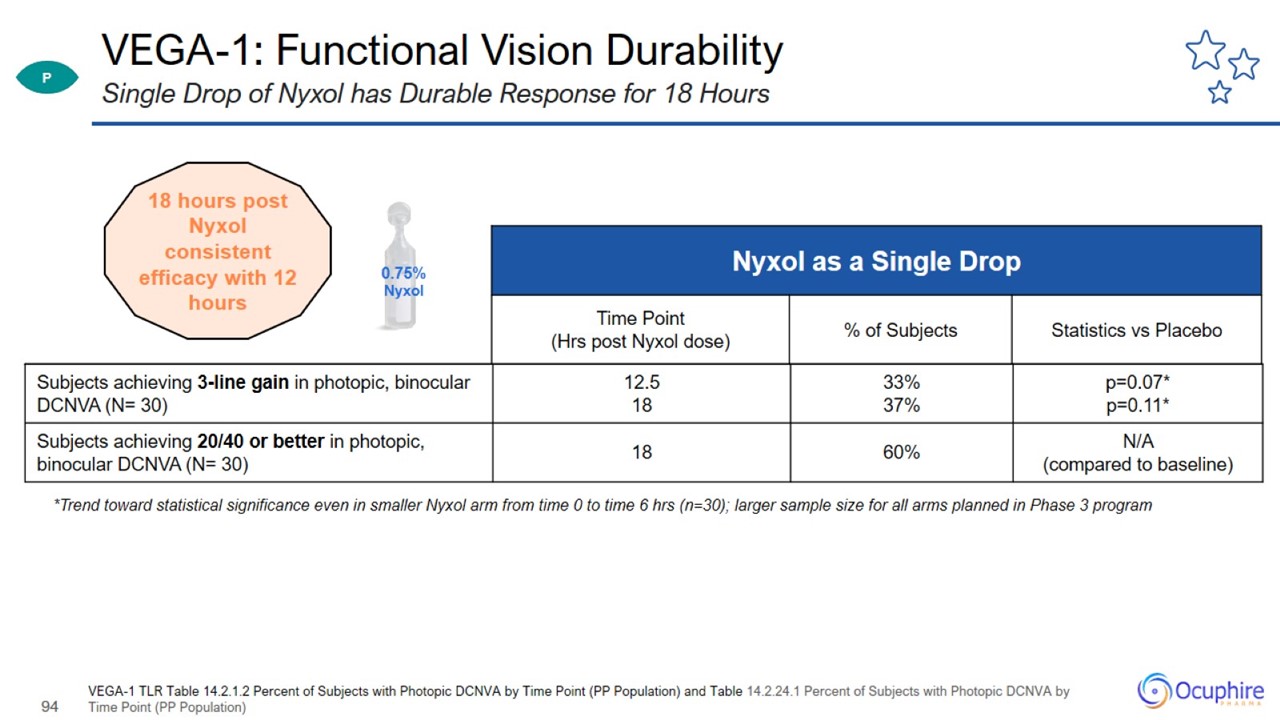
VEGA-1: Functional Vision Durability VEGA-1 TLR Table 14.2.1.2 Percent of Subjects with Photopic DCNVA by Time Point (PP
Population) and Table 14.2.24.1 Percent of Subjects with Photopic DCNVA by Time Point (PP Population) Single Drop of Nyxol has Durable Response for 18 Hours
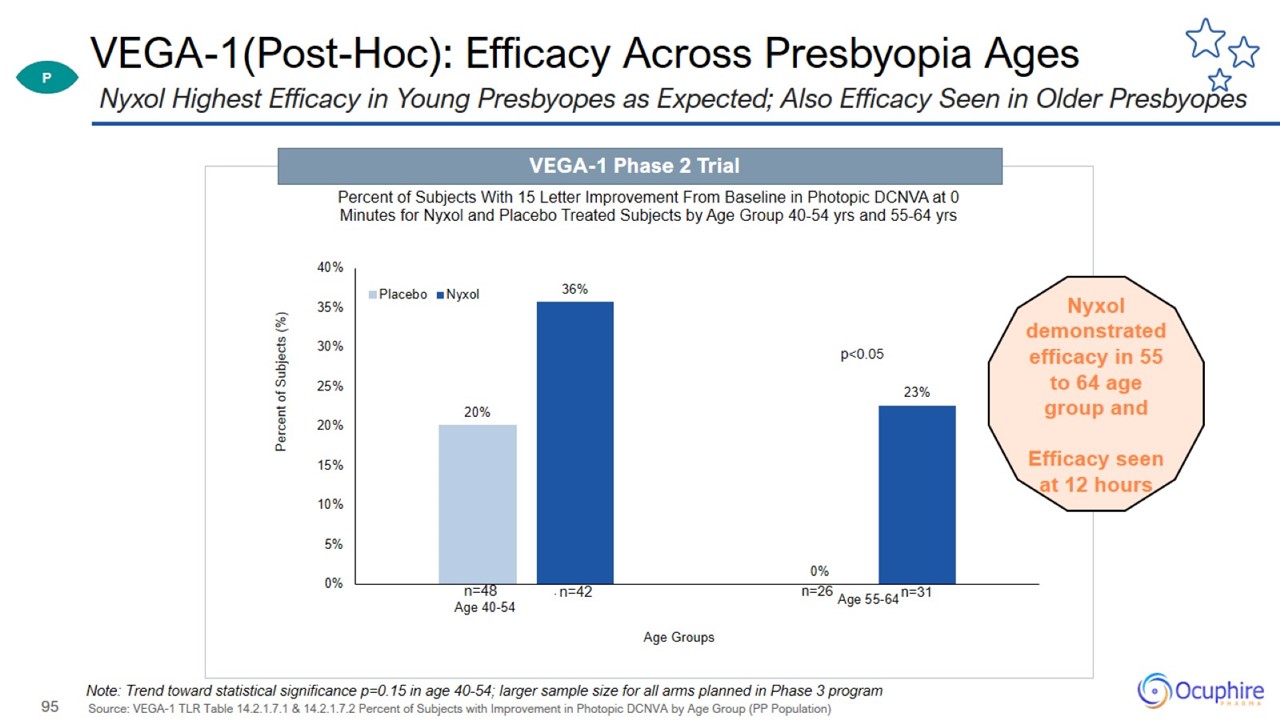
VEGA-1(Post-Hoc): Efficacy Across Presbyopia Ages Source: VEGA-1 TLR Table 14.2.1.7.1 & 14.2.1.7.2 Percent of Subjects with
Improvement in Photopic DCNVA by Age Group (PP Population) Nyxol Highest Efficacy in Young Presbyopes as Expected; Also Efficacy Seen in Older Presbyopes

Presbyopia Drops: Ocuphire Next Steps and Competitive Landscape

Two Treatment Options for Spectrum of Presbyopic Patients Two NDA Submissions Targeted in 2023: Nyxol Alone and Nyxol+LDP
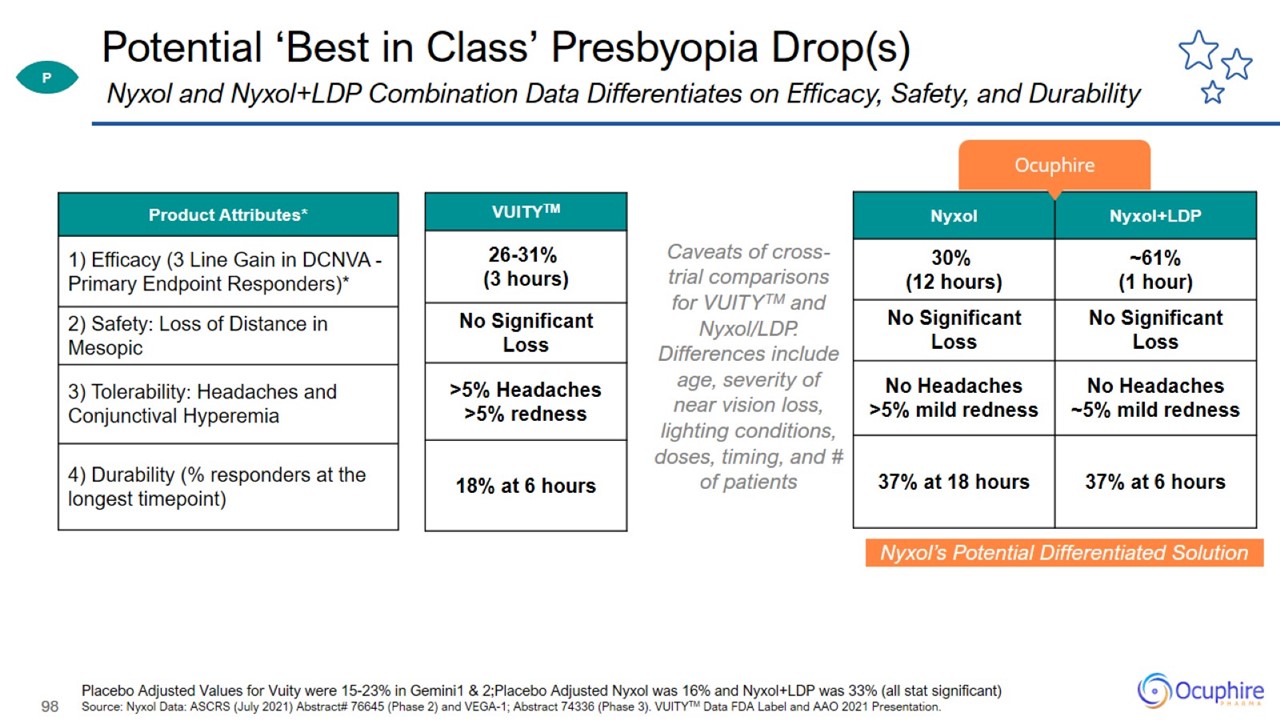
Potential ‘Best in Class’ Presbyopia Drop(s) Placebo Adjusted Values for Vuity were 15-23% in Gemini1 & 2;Placebo Adjusted
Nyxol was 16% and Nyxol+LDP was 33% (all stat significant) Source: Nyxol Data: ASCRS (July 2021) Abstract# 76645 (Phase 2) and VEGA-1; Abstract 74336 (Phase 3). VUITYTM Data FDA Label and AAO 2021 Presentation. Nyxol and Nyxol+LDP Combination
Data Differentiates on Efficacy, Safety, and Durability
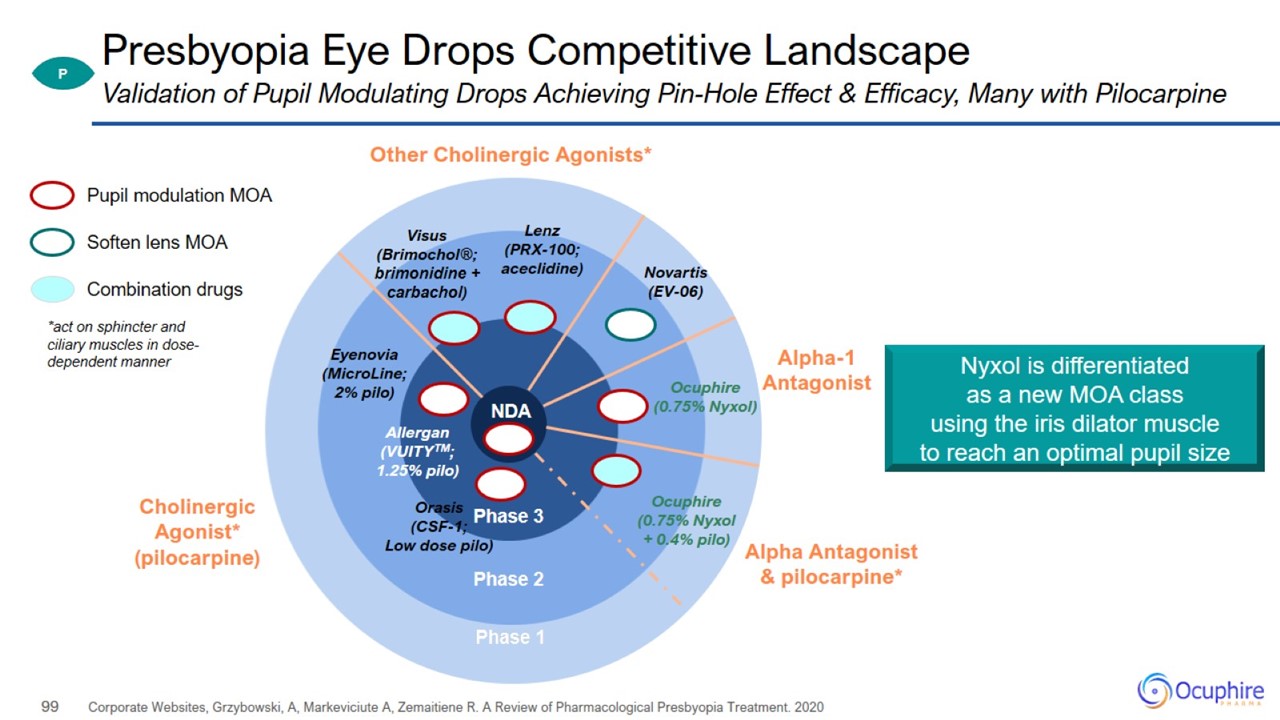
Presbyopia Eye Drops Competitive Landscape Corporate Websites, Grzybowski, A, Markeviciute A, Zemaitiene R. A Review of
Pharmacological Presbyopia Treatment. 2020 Validation of Pupil Modulating Drops Achieving Pin-Hole Effect & Efficacy, Many with Pilocarpine

Large and Growing Presbyopia Market
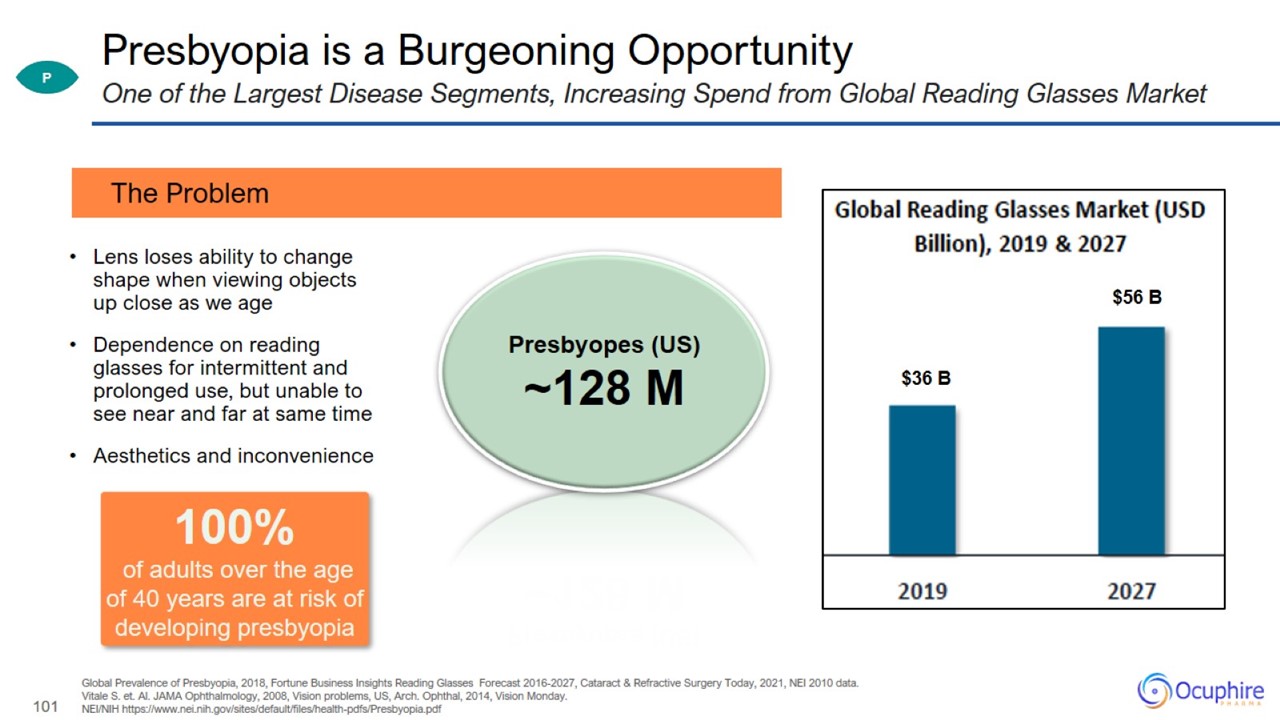
Presbyopia is a Burgeoning Opportunity Global Prevalence of Presbyopia, 2018, Fortune Business Insights Reading Glasses Forecast
2016-2027, Cataract & Refractive Surgery Today, 2021, NEI 2010 data. Vitale S. et. Al. JAMA Ophthalmology, 2008, Vision problems, US, Arch. Ophthal, 2014, Vision Monday. NEI/NIH
https://www.nei.nih.gov/sites/default/files/health-pdfs/Presbyopia.pdf One of the Largest Disease Segments, Increasing Spend from Global Reading Glasses Market
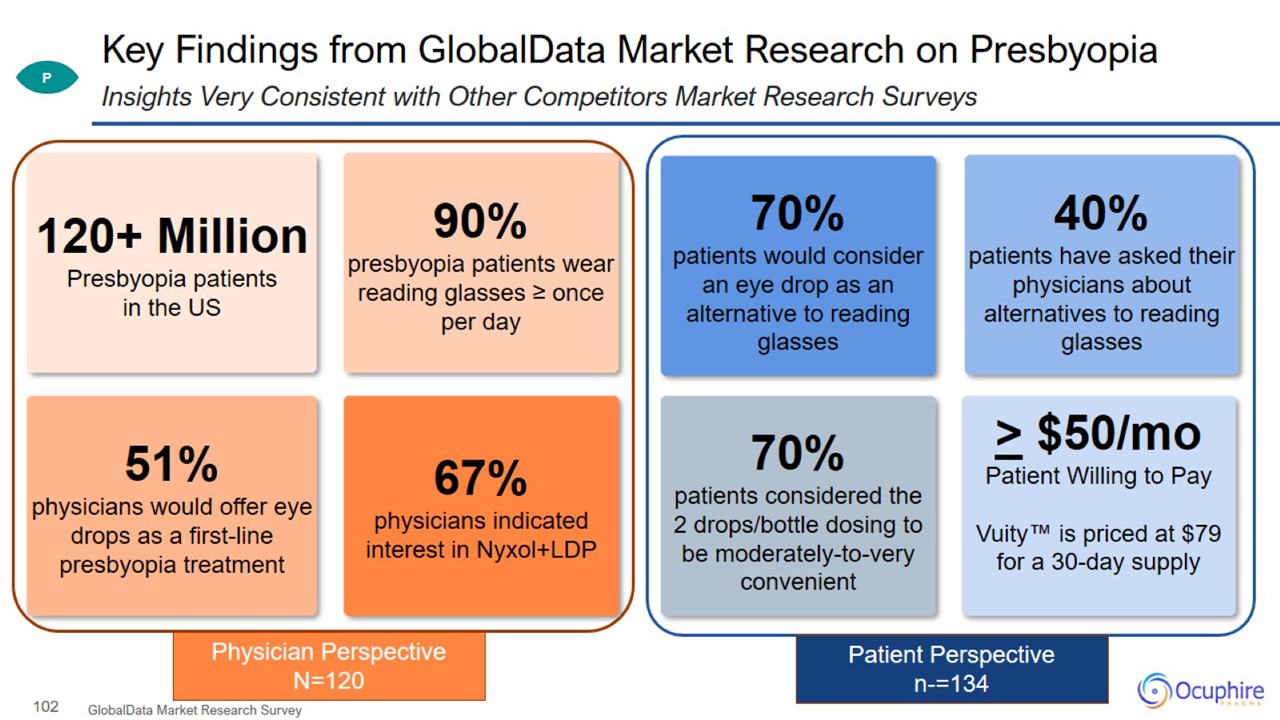
Key Findings from GlobalData Market Research on Presbyopia GlobalData Market Research Survey Insights Very Consistent with Other
Competitors Market Research Surveys
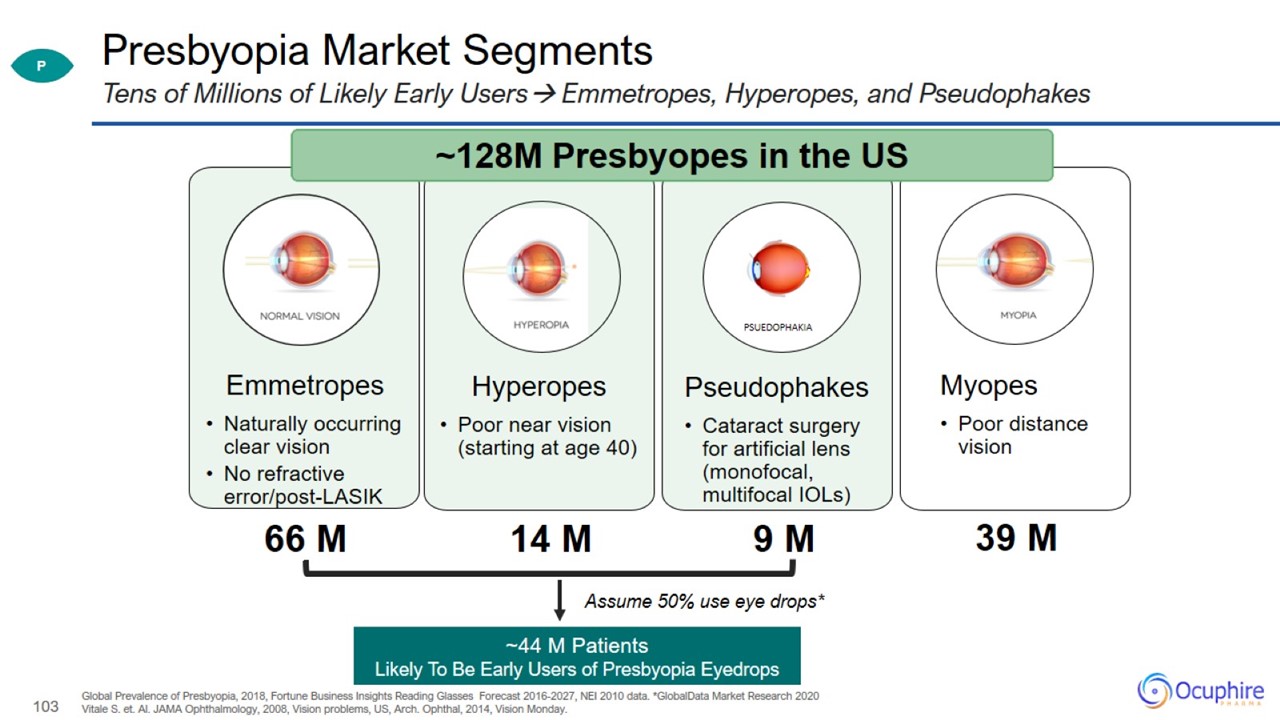
Presbyopia Market Segments Global Prevalence of Presbyopia, 2018, Fortune Business Insights Reading Glasses Forecast 2016-2027,
NEI 2010 data. *GlobalData Market Research 2020 Vitale S. et. Al. JAMA Ophthalmology, 2008, Vision problems, US, Arch. Ophthal, 2014, Vision Monday. Tens of Millions of Likely Early Users Emmetropes, Hyperopes, and Pseudophakes
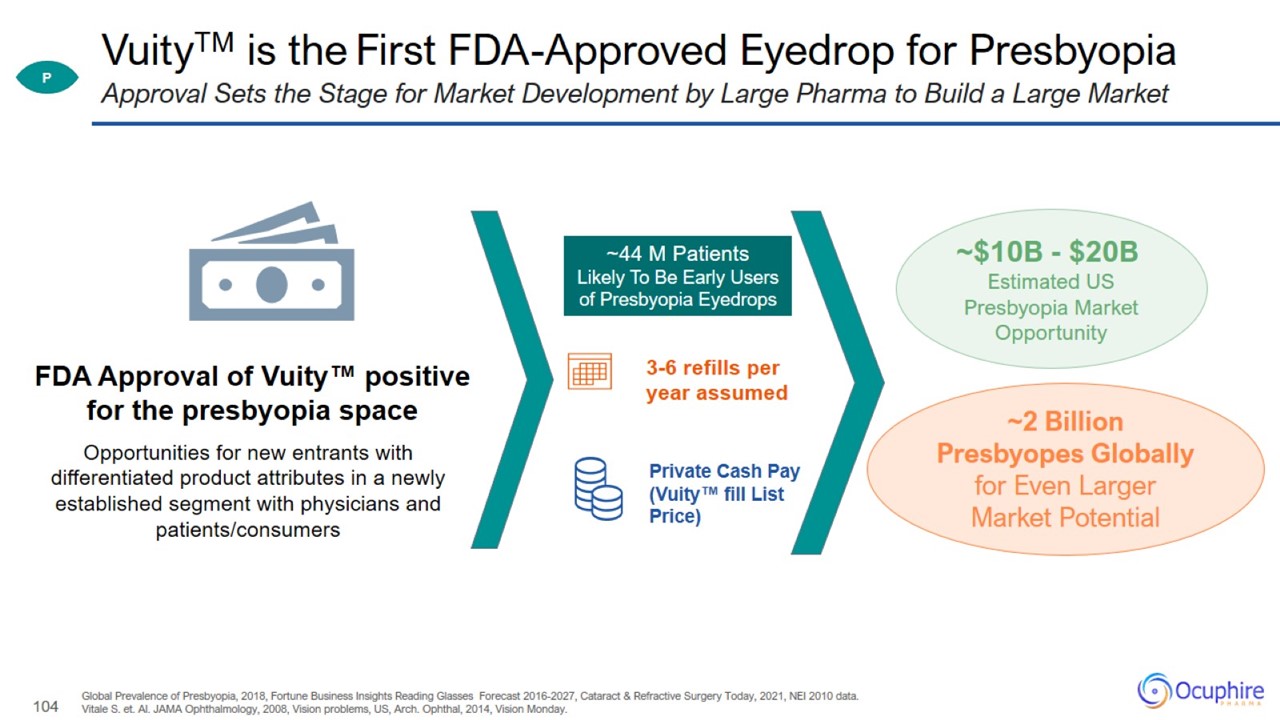
VuityTM is the First FDA-Approved Eyedrop for Presbyopia Global Prevalence of Presbyopia, 2018, Fortune Business Insights Reading
Glasses Forecast 2016-2027, Cataract & Refractive Surgery Today, 2021, NEI 2010 data. Vitale S. et. Al. JAMA Ophthalmology, 2008, Vision problems, US, Arch. Ophthal, 2014, Vision Monday. Approval Sets the Stage for Market Development by
Large Pharma to Build a Large Market

Nyxol® forPRESBYOPIA
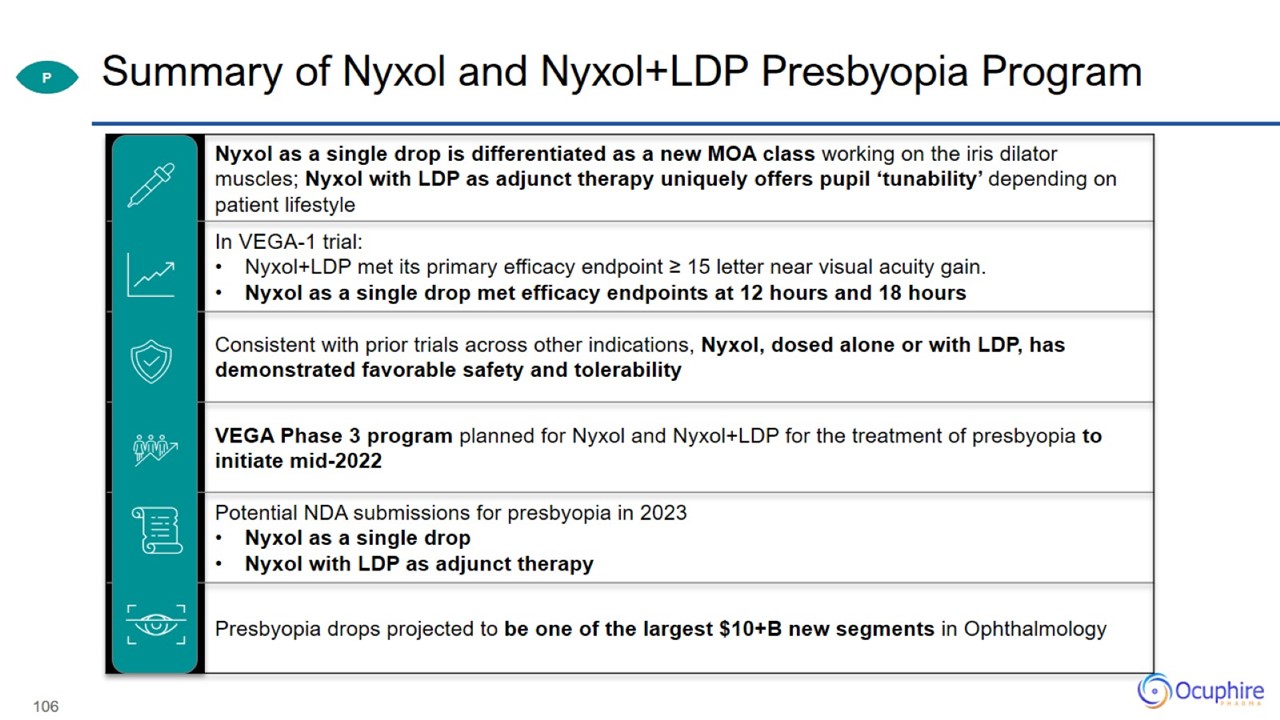
Summary of Nyxol and Nyxol+LDP Presbyopia Program

Closing Remarks Mina Sooch, CEO, Founder of Ocuphire Pharma

Ocuphire Management Team Decades of Biotech and Drug Development Experience

Ocuphire's World-Class Medical Advisory Board Fortunate for the Insights of Leading KOLs & Drug Candidate Co-Founders

Ocuphire Board of Directors
Thanks To Our Network of Partners A Strong Foundation has been Built to Efficiently Grow and Deliver Our Vision for Patients
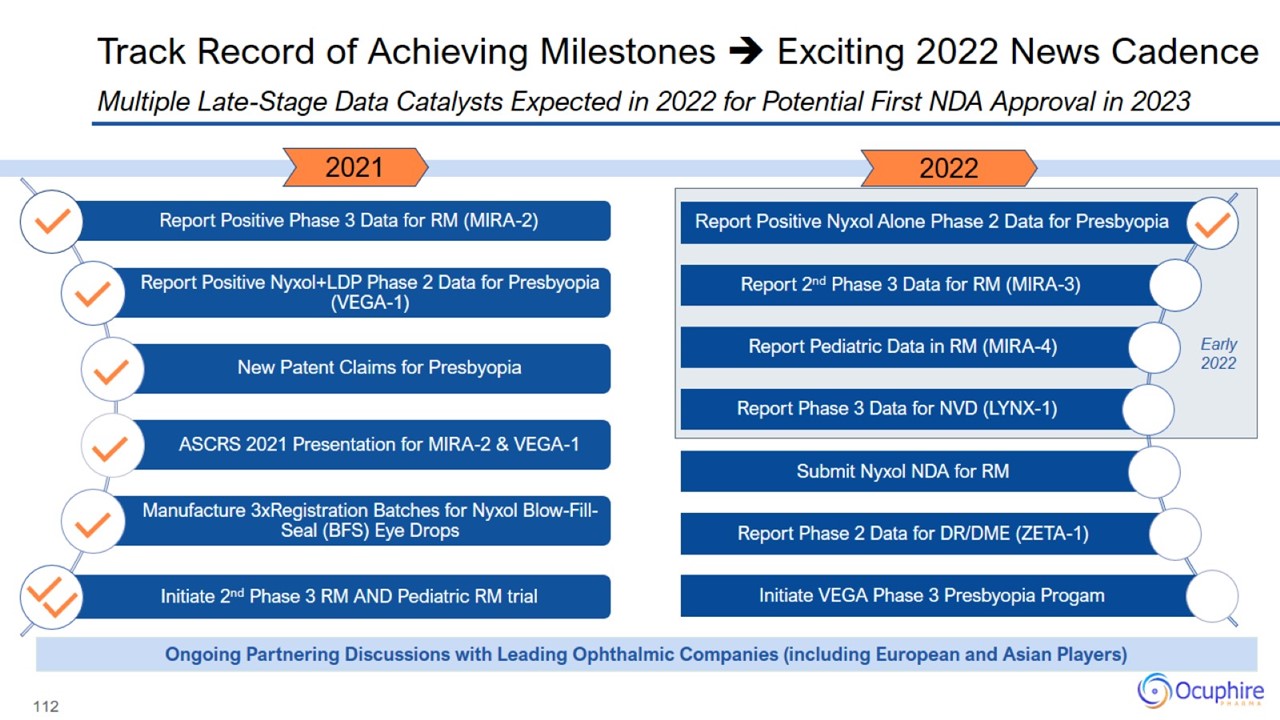
Track Record of Achieving Milestones Exciting 2022 News Cadence
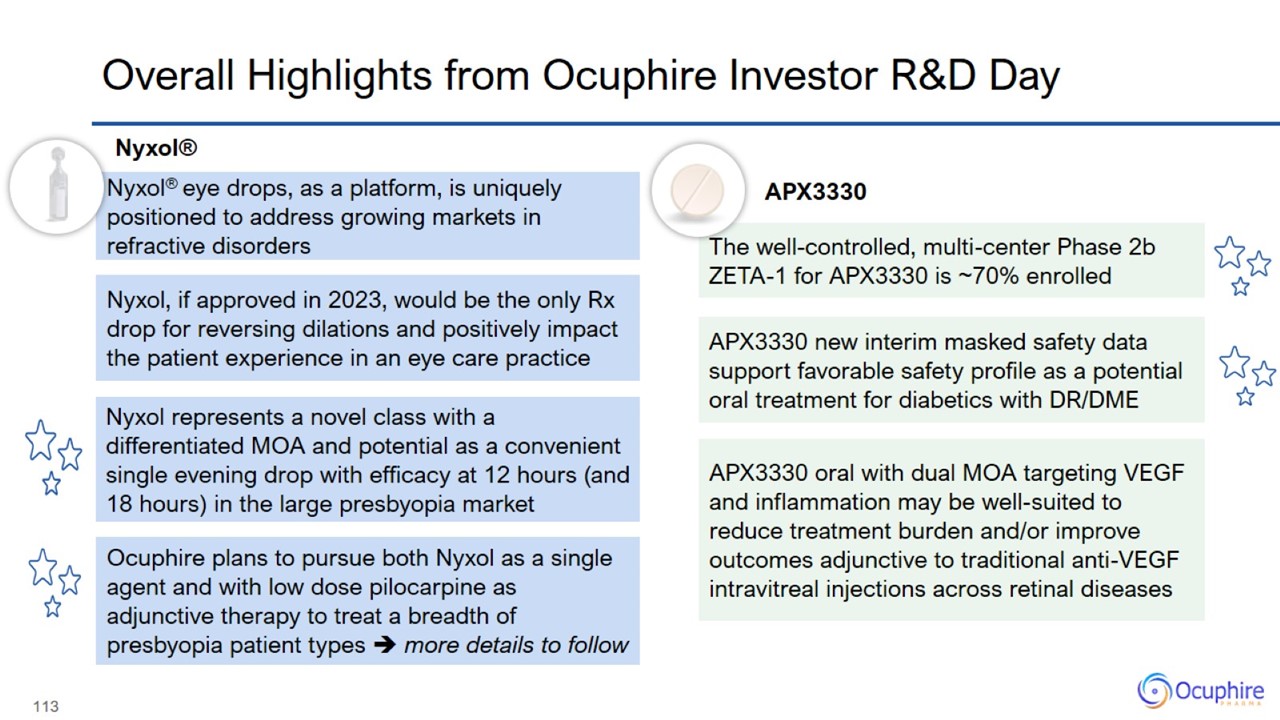
Overall Highlights from Ocuphire Investor R&D Day Nyxol, if approved in 2023, would be the only Rx drop for reversing
dilations and positively impact the patient experience in an eye care practice Nyxol® eye drops, as a platform, is uniquely positioned to address growing markets in refractive disorders

Ocuphire Pharma Investor R&D Day January 31, 2022