EXHIBIT 99.1
Published on March 29, 2022
Exhibit 99.1

MIRA-3 Phase 3 Trial Results Conference Call March 29, 2022

Disclosures and Forward-Looking Statements This presentation contains “forward-looking statements”
within the meaning of the Private Securities Litigation Reform Act of 1995. Such statements include, but are not limited to, statements concerning the regulatory timelines, commercial timelines, cash runway, and future clinical trials in
reversal of mydriasis (RM), presbyopia, night vision disturbance (NVD) and diabetic retinopathy (DR)/diabetic macular edema (DME), and the potential market opportunity in RM. These forward-looking statements are based upon the Company’s
current expectations and involve assumptions that may never materialize or may prove to be incorrect. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of
various risks and uncertainties, including, without limitation: (i) the success and timing of regulatory submissions and pre-clinical and clinical trials, including enrollment and data readouts; (ii) regulatory requirements or developments;
(iii) changes to clinical trial designs and regulatory pathways; (iv) changes in capital resource requirements; (v) risks related to the inability of Ocuphire to obtain sufficient additional capital to continue to advance its product
candidates and its preclinical programs; (vi) legislative, regulatory, political and economic developments, (vii) changes in market opportunities, (viii) the effects of COVID-19 on clinical programs and business operations, (ix) the success
and timing of commercialization of any of Ocuphire’s product candidates and (x) the maintenance of Ocuphire’s intellectual property rights. The foregoing review of important factors that could cause actual events to differ from expectations
should not be construed as exhaustive and should be read in conjunction with statements that are included herein and elsewhere, including the risk factors detailed in documents that have been and may be filed by the Company from time to time
with the SEC. All forward-looking statements contained in this presentation speak only as of the date on which they were made. The Company undertakes no obligation to update such statements to reflect events that occur or circumstances that
exist after the date on which they were made. The Company makes no representation or warranty, express or implied, as to the accuracy or completeness of the information contained in or incorporated by reference into this presentation.
Nothing contained in or incorporated by reference into this presentation is, or shall be relied upon as, a promise or representation by the Company as to the past or future. The Company assumes no responsibility for the accuracy or
completeness of any such information. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market shares and other data about our industry. This data involves a number of
assumptions and limitations, and you are cautioned not to give undue weight to such estimates. The trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use should not be construed
as an endorsement of such products.
Agenda and Participants Highlights and Overview Topline MIRA-3 Phase 3 Clinical Trial Results for
Nyxol in Reversal of Mydriasis (RM) Reversal of Mydriasis Market Opportunity Upcoming Milestones Q&A Participants Mina Sooch, MBA, President and CEO Jay Pepose, MD, PhD, Medical Advisory Board and Board Member Mitch Brigell, PhD,
Head of Clinical Development Susan Benton, MBA, Corporate Board Member Bindu Manne, Head of Market Development and Commercialization Charlie Hoffmann, MBA, VP of Corporate Development and Operations Amy Rabourn, MAcc, VP of
Finance Second Phase 3 RM Trial Topline Readout as Planned in 1Q22

Highlights and Overview
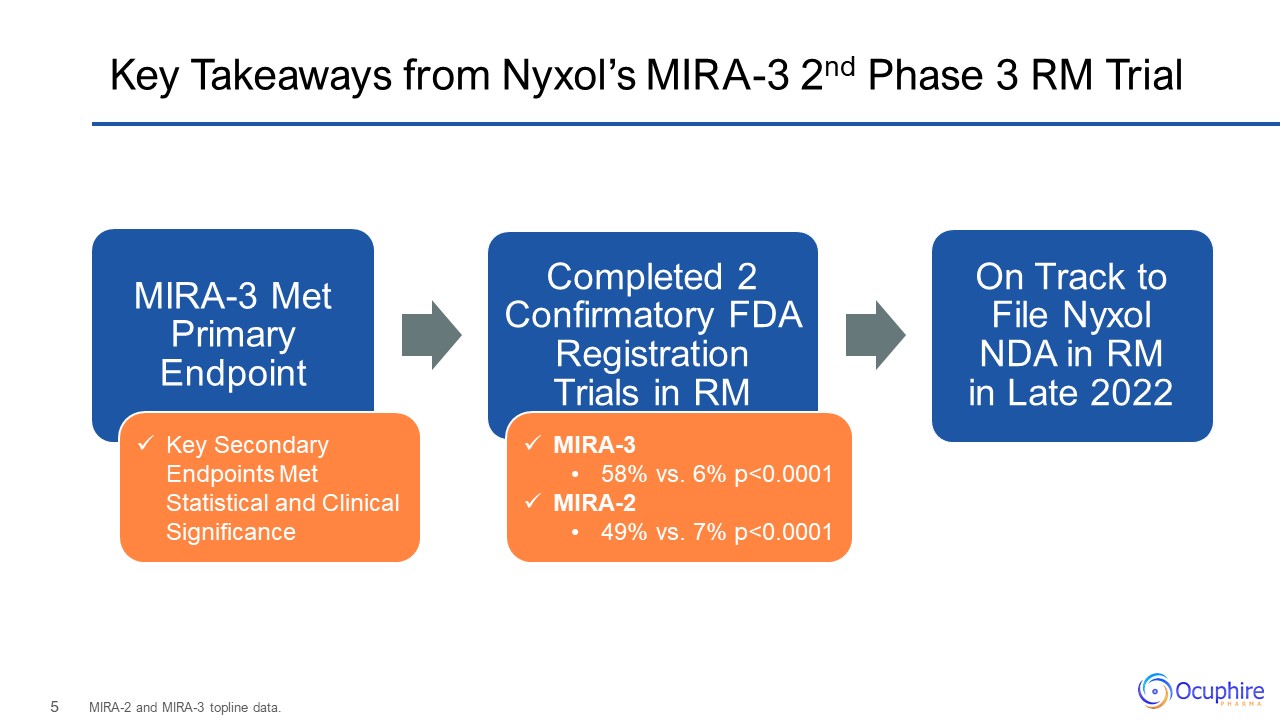
Key Takeaways from Nyxol’s MIRA-3 2nd Phase 3 RM Trial MIRA-2 and MIRA-3 topline data. MIRA-3 58%
vs. 6% p<0.0001 MIRA-2 49% vs. 7% p<0.0001 Key Secondary Endpoints Met Statistical and Clinical Significance
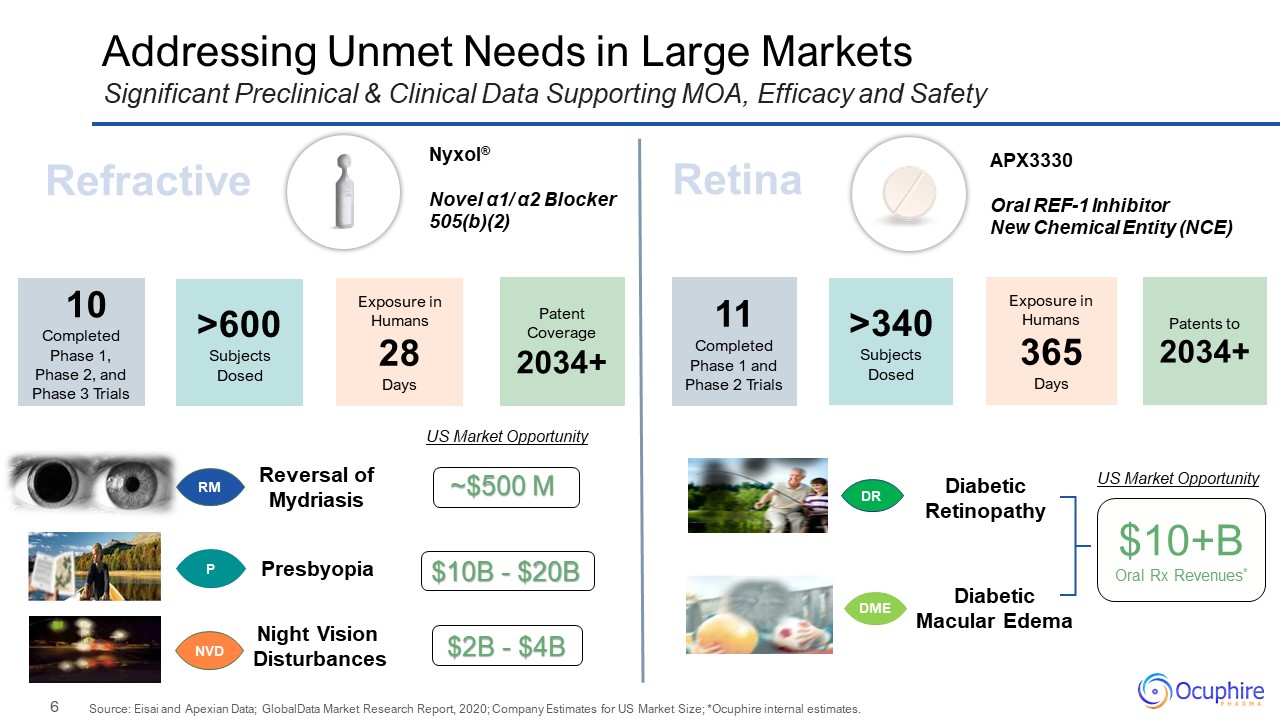
Addressing Unmet Needs in Large Markets Source: Eisai and Apexian Data; GlobalData Market Research
Report, 2020; Company Estimates for US Market Size; *Ocuphire internal estimates. Significant Preclinical & Clinical Data Supporting MOA, Efficacy and Safety 11 Completed Phase 1 and Phase 2 Trials >340Subjects Dosed Patents
to2034+ APX3330 Oral REF-1 Inhibitor New Chemical Entity (NCE) Nyxol® Novel α1/ α2 Blocker 505(b)(2) 10 Completed Phase 1,Phase 2, and Phase 3 Trials >600Subjects Dosed Exposure in Humans 28 Days Patent
Coverage2034+ Exposure in Humans 365 Days Refractive Retina Presbyopia Reversal of Mydriasis Night Vision Disturbances Diabetic Retinopathy Diabetic Macular Edema DR DME RM P NVD US Market Opportunity $2B - $4B $10B -
$20B ~$500 M US Market Opportunity $10+B Oral Rx Revenues*
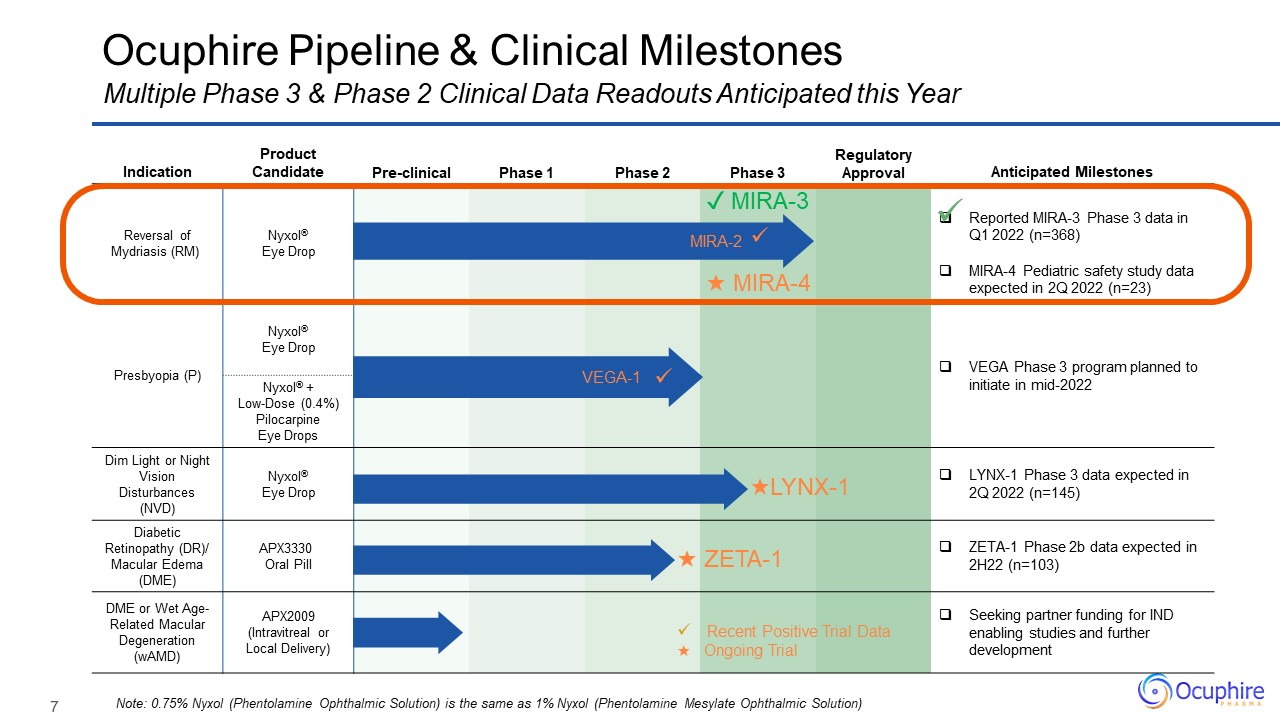
Indication Product Candidate Pre-clinical Phase 1 Phase 2 Phase
3 Regulatory Approval Anticipated Milestones Reversal of Mydriasis (RM) Nyxol® Eye Drop Reported MIRA-3 Phase 3 data in Q1 2022 (n=368) MIRA-4 Pediatric safety study data expected in 2Q 2022 (n=23) Presbyopia (P) Nyxol® Eye
Drop VEGA Phase 3 program planned to initiate in mid-2022 Presbyopia (P) Nyxol® + Low-Dose (0.4%) Pilocarpine Eye Drops Dim Light or Night Vision Disturbances (NVD) Nyxol® Eye Drop LYNX-1 Phase 3 data expected in 2Q 2022
(n=145) Diabetic Retinopathy (DR)/ Macular Edema (DME) APX3330 Oral Pill ZETA-1 Phase 2b data expected in 2H22 (n=103) DME or Wet Age-Related Macular Degeneration (wAMD) APX2009 (Intravitreal or Local Delivery) Seeking partner
funding for IND enabling studies and further development ✓ MIRA-3 Ocuphire Pipeline & Clinical Milestones Note: 0.75% Nyxol (Phentolamine Ophthalmic Solution) is the same as 1% Nyxol (Phentolamine Mesylate Ophthalmic
Solution) Multiple Phase 3 & Phase 2 Clinical Data Readouts Anticipated this Year MIRA-2 VEGA-1 Recent Positive Trial Data ★ Ongoing Trial ★ ZETA-1 ★LYNX-1 ★ MIRA-4
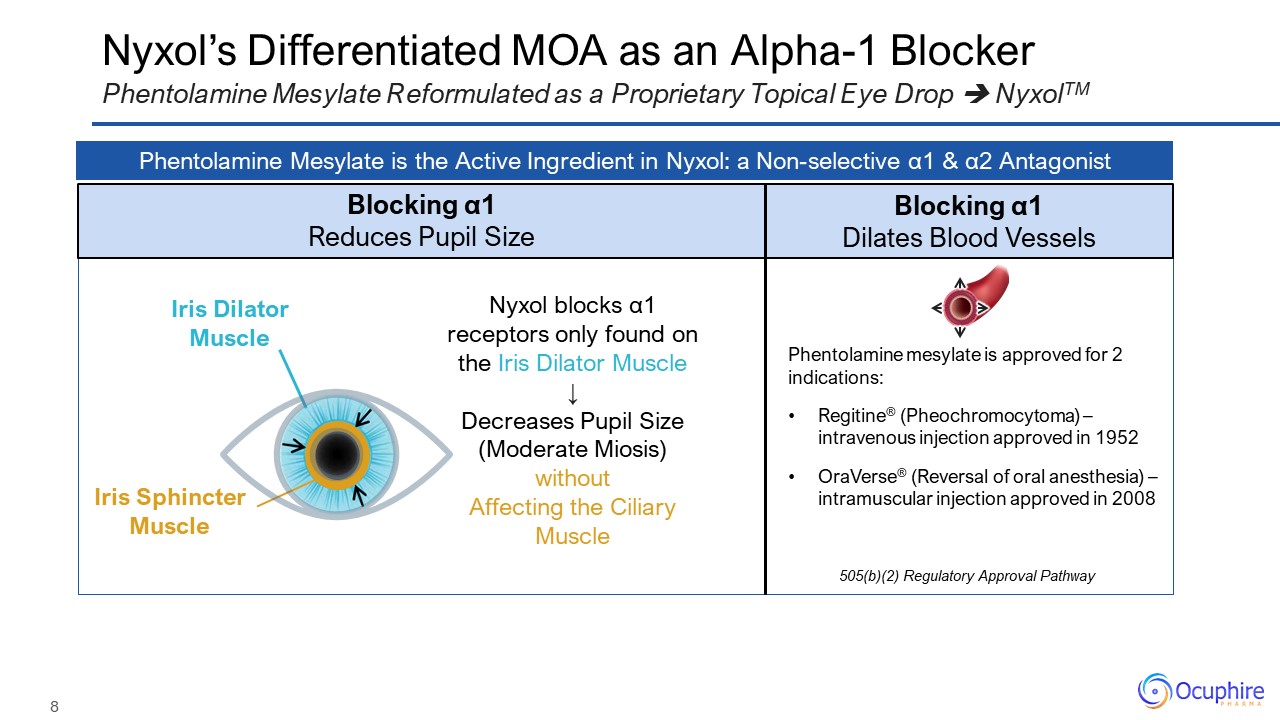
Nyxol’s Differentiated MOA as an Alpha-1 Blocker Phentolamine Mesylate Reformulated as a Proprietary
Topical Eye Drop NyxolTM Phentolamine Mesylate is the Active Ingredient in Nyxol: a Non-selective α1 & α2 Antagonist Blocking α1 Reduces Pupil Size Blocking α1 Dilates Blood Vessels Phentolamine mesylate is approved for 2
indications: Regitine® (Pheochromocytoma) – intravenous injection approved in 1952 OraVerse® (Reversal of oral anesthesia) – intramuscular injection approved in 2008 505(b)(2) Regulatory Approval Pathway Nyxol blocks α1 receptors only
found on the Iris Dilator Muscle ↓ Decreases Pupil Size (Moderate Miosis) without Affecting the Ciliary Muscle Iris Dilator Muscle Iris Sphincter Muscle
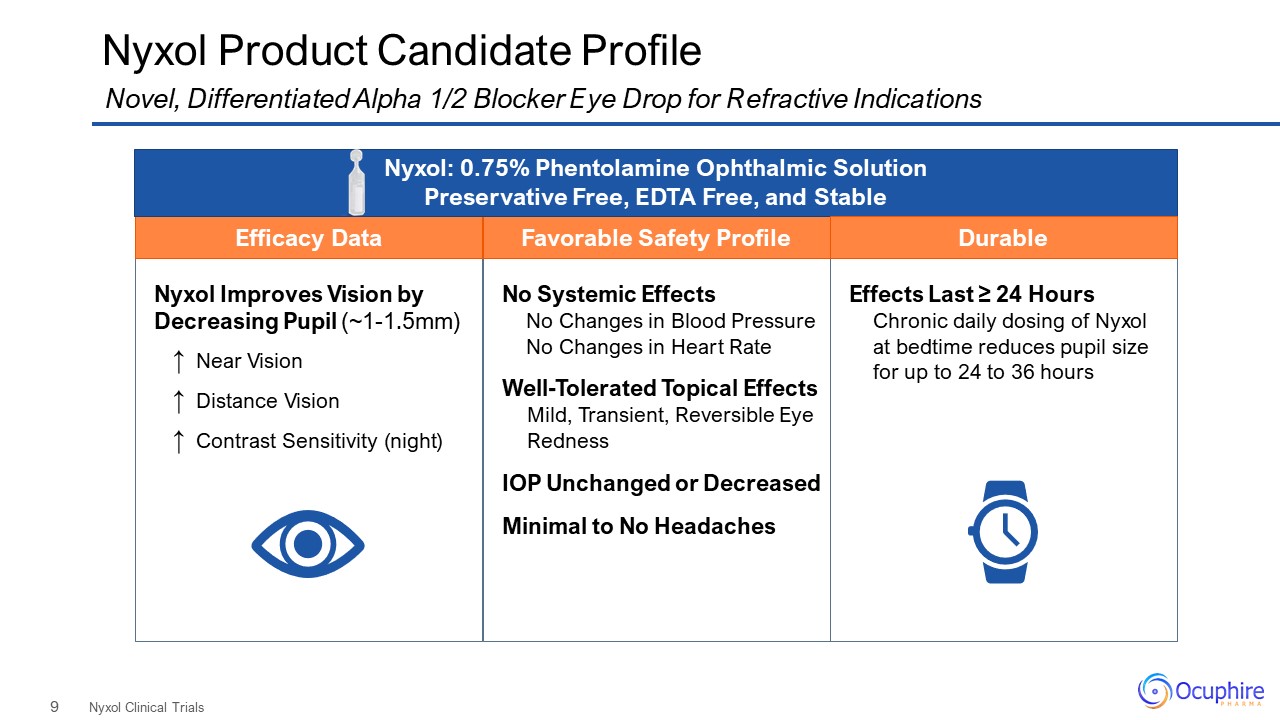
Nyxol Improves Vision by Decreasing Pupil (~1-1.5mm) ↑ Near Vision ↑ Distance Vision ↑ Contrast
Sensitivity (night) No Systemic Effects No Changes in Blood Pressure No Changes in Heart Rate Well-Tolerated Topical Effects Mild, Transient, Reversible Eye Redness IOP Unchanged or Decreased Minimal to No Headaches Nyxol Product
Candidate Profile Nyxol Clinical Trials Novel, Differentiated Alpha 1/2 Blocker Eye Drop for Refractive Indications Favorable Safety Profile Efficacy Data Nyxol: 0.75% Phentolamine Ophthalmic Solution Preservative Free, EDTA Free, and
Stable Effects Last ≥ 24 Hours Chronic daily dosing of Nyxol at bedtime reduces pupil size for up to 24 to 36 hours Durable
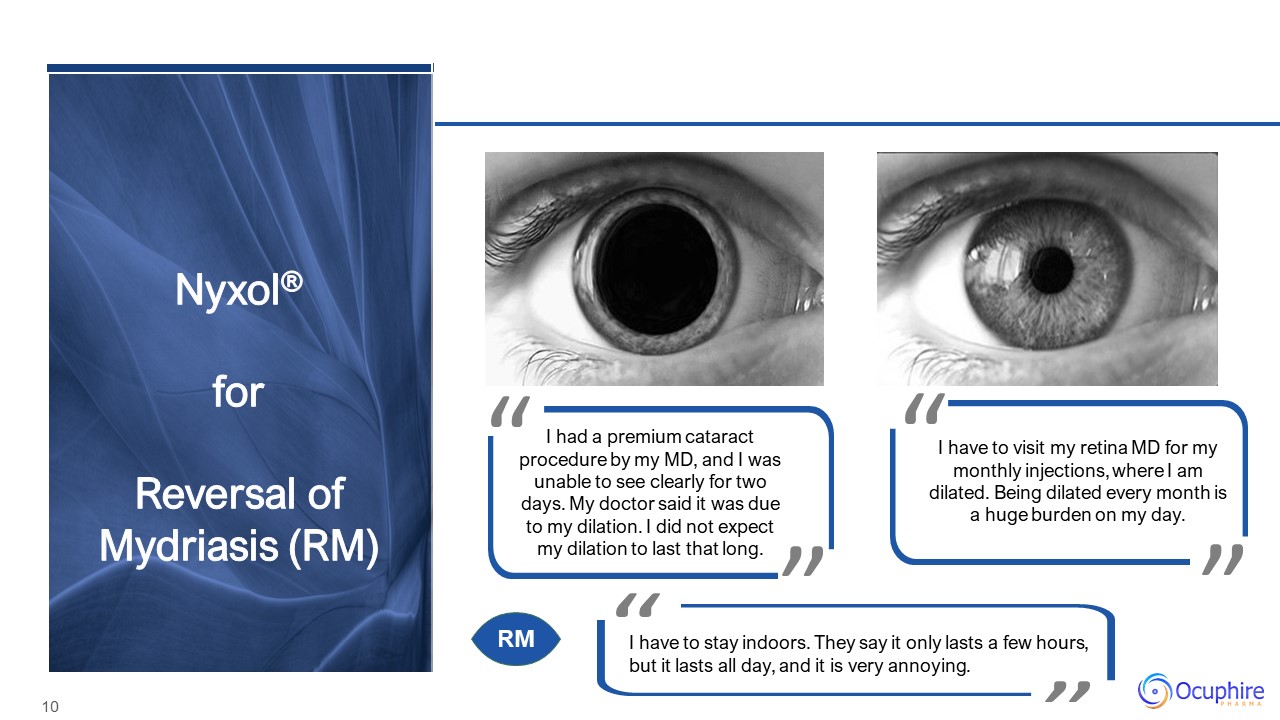
Nyxol®forReversal of Mydriasis (RM) I have to visit my retina MD for my monthly injections, where I am
dilated. Being dilated every month is a huge burden on my day. I had a premium cataract procedure by my MD, and I was unable to see clearly for two days. My doctor said it was due to my dilation. I did not
expect my dilation to last that long. I have to stay indoors. They say it only lasts a few hours, but it lasts all day, and it is very annoying. RM
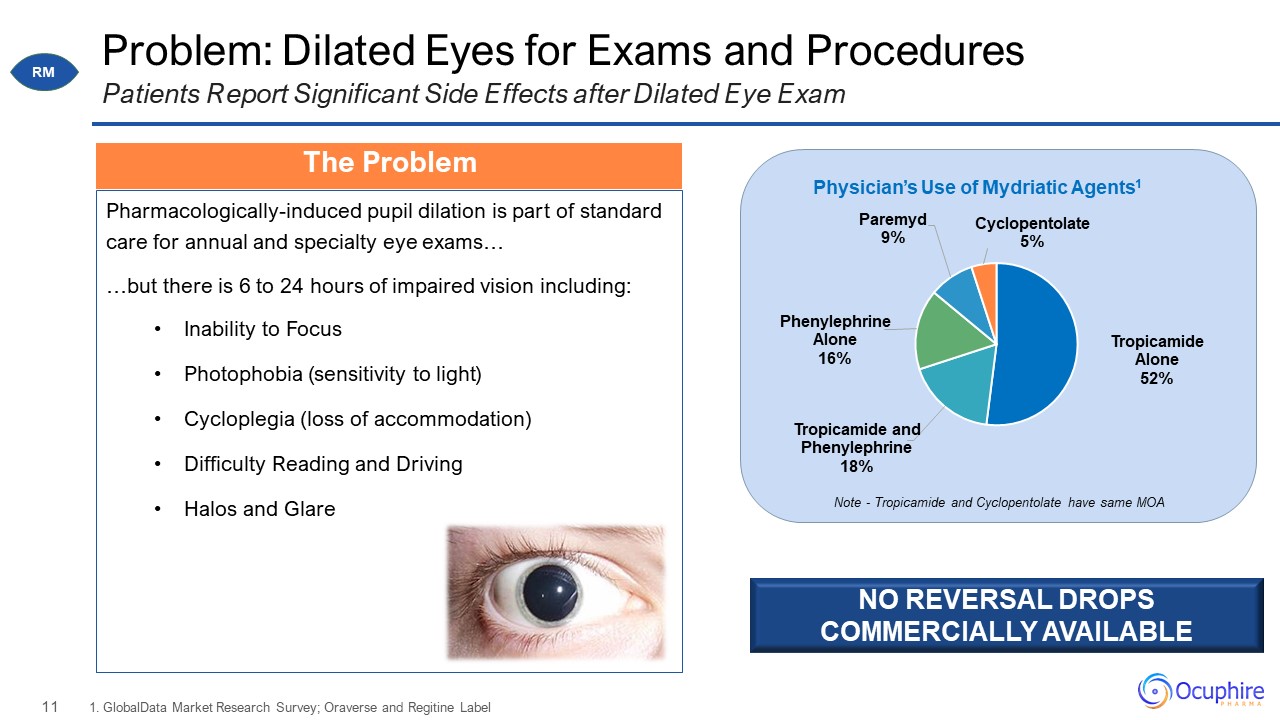
Problem: Dilated Eyes for Exams and Procedures Pharmacologically-induced pupil dilation is part of
standard care for annual and specialty eye exams… …but there is 6 to 24 hours of impaired vision including: Inability to Focus Photophobia (sensitivity to light) Cycloplegia (loss of accommodation) Difficulty Reading and
Driving Halos and Glare 1. GlobalData Market Research Survey; Oraverse and Regitine Label Patients Report Significant Side Effects after Dilated Eye Exam Note - Tropicamide and Cyclopentolate have same MOA NO REVERSAL DROPS COMMERCIALLY
AVAILABLE The Problem RM
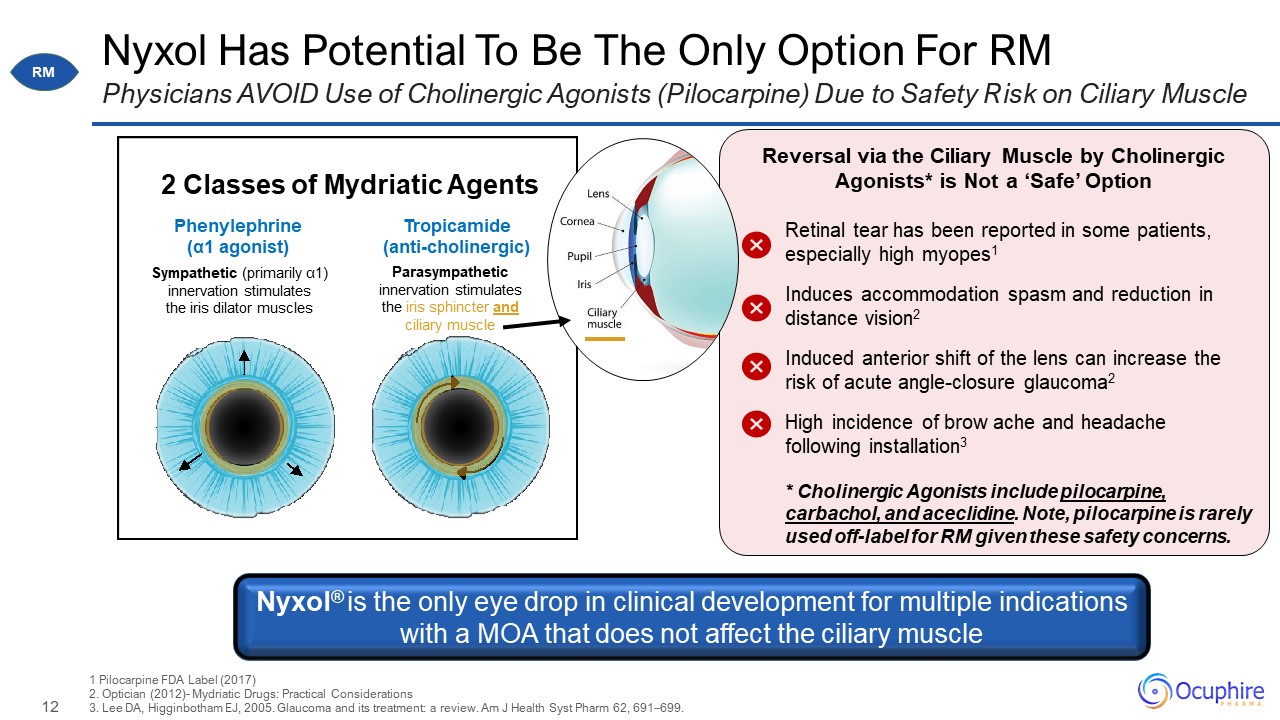
Nyxol Has Potential To Be The Only Option For RM Physicians AVOID Use of Cholinergic Agonists
(Pilocarpine) Due to Safety Risk on Ciliary Muscle 1 Pilocarpine FDA Label (2017)2. Optician (2012)- Mydriatic Drugs: Practical Considerations 3. Lee DA, Higginbotham EJ, 2005. Glaucoma and its treatment: a review. Am J Health Syst Pharm
62, 691–699. Parasympathetic innervation stimulates the iris sphincter and ciliary muscle Sympathetic (primarily α1) innervation stimulates the iris dilator muscles 2 Classes of Mydriatic Agents Tropicamide
(anti-cholinergic) Phenylephrine (α1 agonist) Reversal via the Ciliary Muscle by Cholinergic Agonists* is Not a ‘Safe’ Option Retinal tear has been reported in some patients, especially high myopes1 Induces accommodation spasm and
reduction in distance vision2 Induced anterior shift of the lens can increase the risk of acute angle-closure glaucoma2 High incidence of brow ache and headache following installation3 * Cholinergic Agonists include pilocarpine, carbachol,
and aceclidine. Note, pilocarpine is rarely used off-label for RM given these safety concerns. Nyxol® is the only eye drop in clinical development for multiple indications with a MOA that does not affect the ciliary muscle RM

MIRA-3 Topline Phase 3 Results Randomized, Parallel Arm, Double-Masked, Placebo-Controlled Study of
the Safety and Efficacy of Nyxol (0.75% Phentolamine Ophthalmic Solution) to Reverse Pharmacologically-Induced Mydriasis in Healthy Subjects
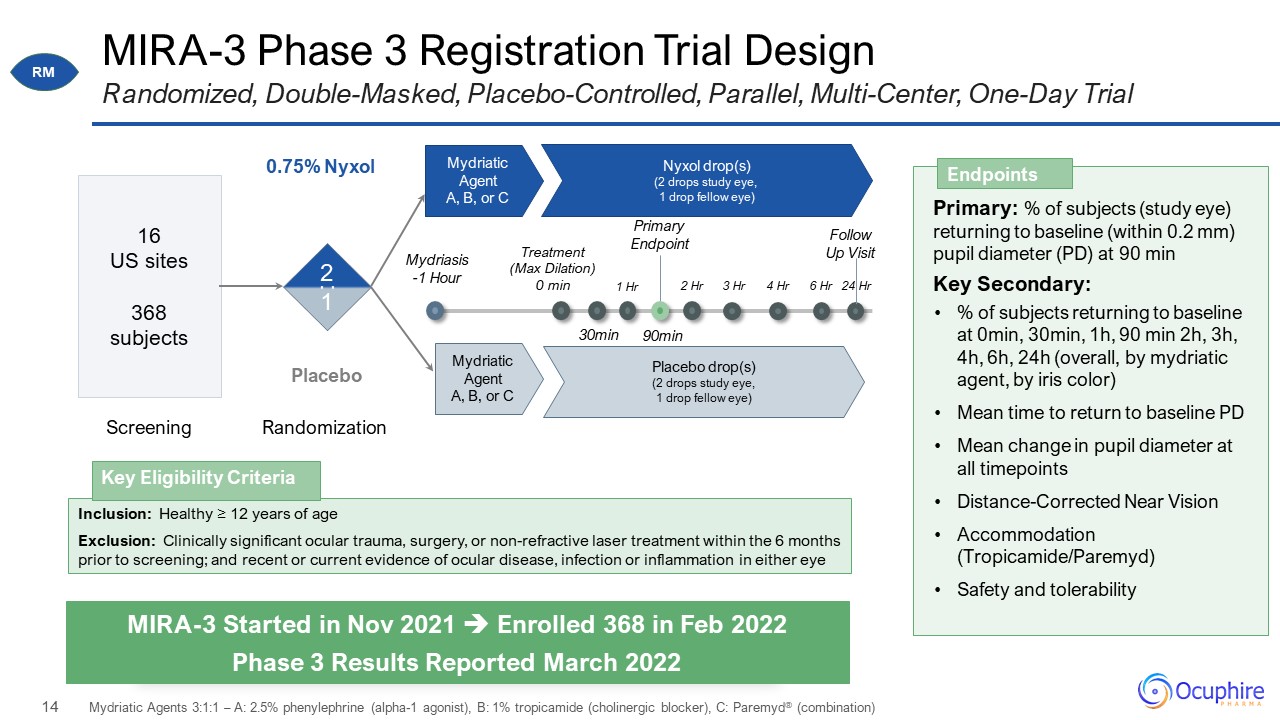
MIRA-3 Phase 3 Registration Trial Design Randomized, Double-Masked, Placebo-Controlled, Parallel,
Multi-Center, One-Day Trial Mydriasis -1 Hour 1:1 Mydriatic Agent A, B, or C 0.75% Nyxol Placebo 16 US sites 368 subjects Nyxol drop(s) (2 drops study eye, 1 drop fellow eye) Mydriatic Agent A, B, or C Placebo drop(s) (2
drops study eye, 1 drop fellow eye) Primary: % of subjects (study eye) returning to baseline (within 0.2 mm) pupil diameter (PD) at 90 min Key Secondary: % of subjects returning to baseline at 0min, 30min, 1h, 90 min 2h, 3h, 4h, 6h, 24h
(overall, by mydriatic agent, by iris color) Mean time to return to baseline PD Mean change in pupil diameter at all timepoints Distance-Corrected Near Vision Accommodation (Tropicamide/Paremyd) Safety and
tolerability Endpoints MIRA-3 Started in Nov 2021 Enrolled 368 in Feb 2022 Phase 3 Results Reported March 2022 Screening Randomization 2 1 Treatment (Max Dilation) 0 min 30min 1 Hr 90min 2 Hr 3 Hr 4 Hr 6 Hr 24
Hr Primary Endpoint Follow Up Visit Mydriatic Agents 3:1:1 – A: 2.5% phenylephrine (alpha-1 agonist), B: 1% tropicamide (cholinergic blocker), C: Paremyd® (combination) : Inclusion: Healthy ≥ 12 years of age Exclusion: Clinically
significant ocular trauma, surgery, or non-refractive laser treatment within the 6 months prior to screening; and recent or current evidence of ocular disease, infection or inflammation in either eye Key Eligibility Criteria RM
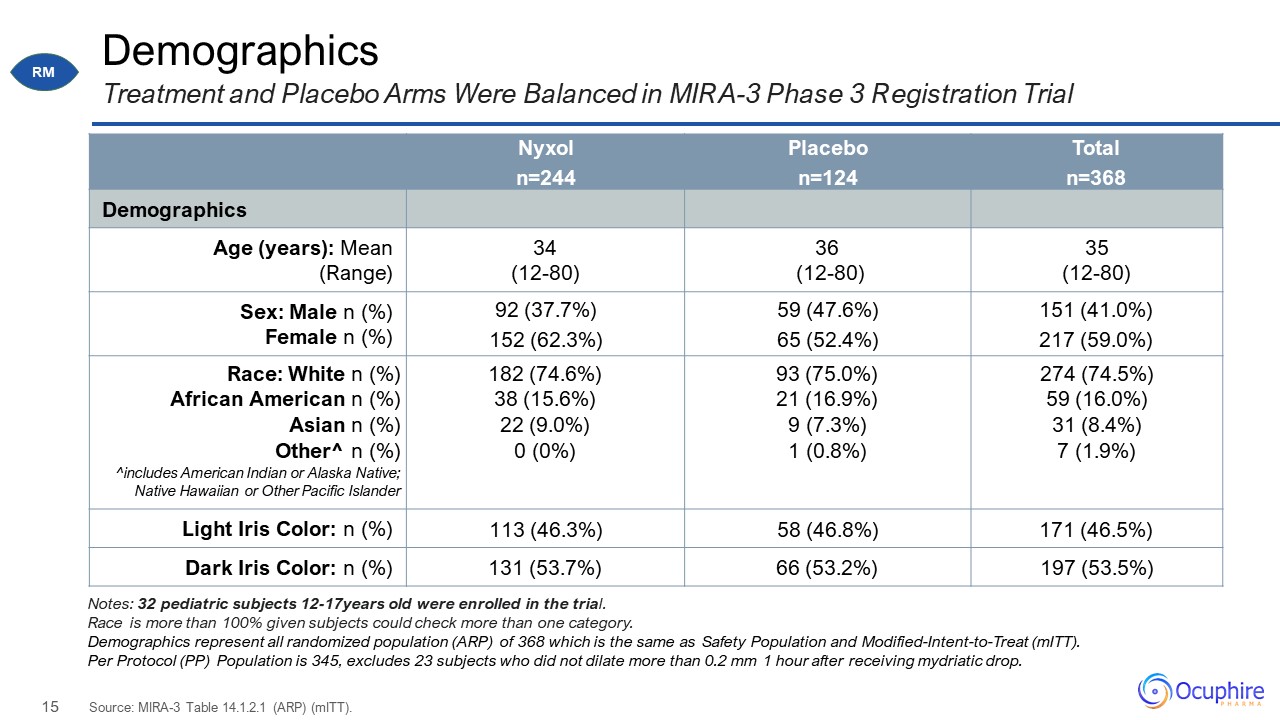
Demographics Nyxol n=244 Placebo n=124 Total n=368 Demographics Age (years): Mean (Range) 34
(12-80) 36 (12-80) 35 (12-80) Sex: Male n (%) Female n (%) 92 (37.7%) 152 (62.3%) 59 (47.6%) 65 (52.4%) 151 (41.0%) 217 (59.0%) Race: White n (%) African American n (%) Asian n (%) Other^ n (%) ^includes American Indian
or Alaska Native; Native Hawaiian or Other Pacific Islander 182 (74.6%) 38 (15.6%) 22 (9.0%) 0 (0%) 93 (75.0%) 21 (16.9%) 9 (7.3%) 1 (0.8%) 274 (74.5%) 59 (16.0%) 31 (8.4%) 7 (1.9%) Light Iris Color: n (%) 113 (46.3%) 58
(46.8%) 171 (46.5%) Dark Iris Color: n (%) 131 (53.7%) 66 (53.2%) 197 (53.5%) Treatment and Placebo Arms Were Balanced in MIRA-3 Phase 3 Registration Trial Source: MIRA-3 Table 14.1.2.1 (ARP) (mITT). Notes: 32 pediatric subjects
12-17years old were enrolled in the trial. Race is more than 100% given subjects could check more than one category. Demographics represent all randomized population (ARP) of 368 which is the same as Safety Population and
Modified-Intent-to-Treat (mITT). Per Protocol (PP) Population is 345, excludes 23 subjects who did not dilate more than 0.2 mm 1 hour after receiving mydriatic drop. RM
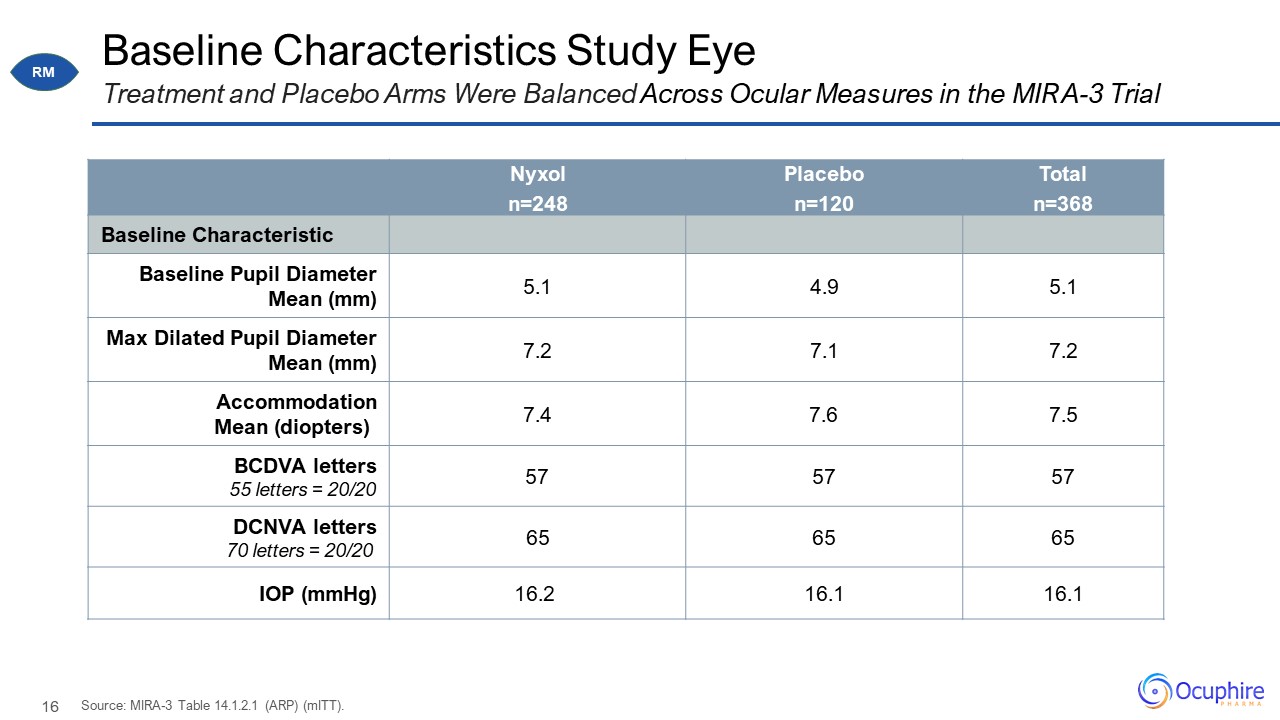
Baseline Characteristics Study Eye Nyxol n=248 Placebo n=120 Total n=368 Baseline
Characteristic Baseline Pupil Diameter Mean (mm) 5.1 4.9 5.1 Max Dilated Pupil Diameter Mean (mm) 7.2 7.1 7.2 Accommodation Mean (diopters) 7.4 7.6 7.5 BCDVA letters 55 letters = 20/20 57 57 57 DCNVA letters 70 letters =
20/20 65 65 65 IOP (mmHg) 16.2 16.1 16.1 Treatment and Placebo Arms Were Balanced Across Ocular Measures in the MIRA-3 Trial Source: MIRA-3 Table 14.1.2.1 (ARP) (mITT). RM
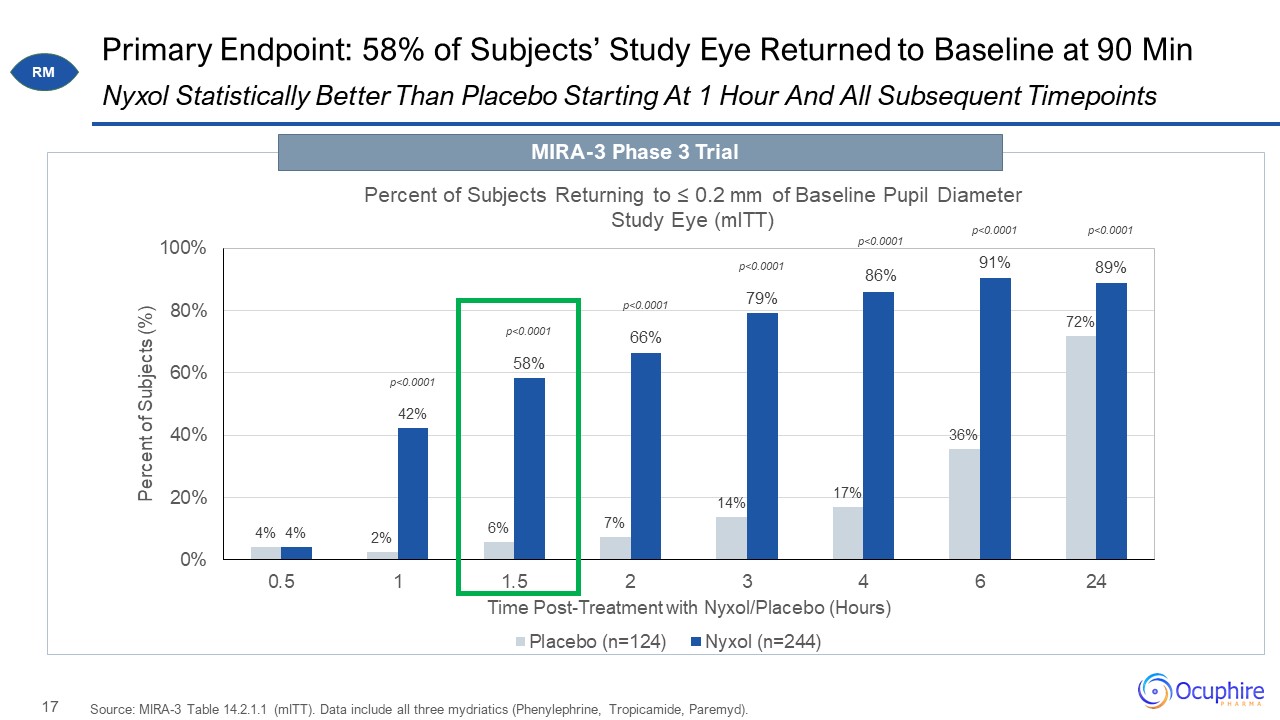
Primary Endpoint: 58% of Subjects’ Study Eye Returned to Baseline at 90 Min Source: MIRA-3 Table
14.2.1.1 (mITT). Data include all three mydriatics (Phenylephrine, Tropicamide, Paremyd). Nyxol Statistically Better Than Placebo Starting At 1 Hour And All Subsequent Timepoints MIRA-3 Phase 3 Trial RM
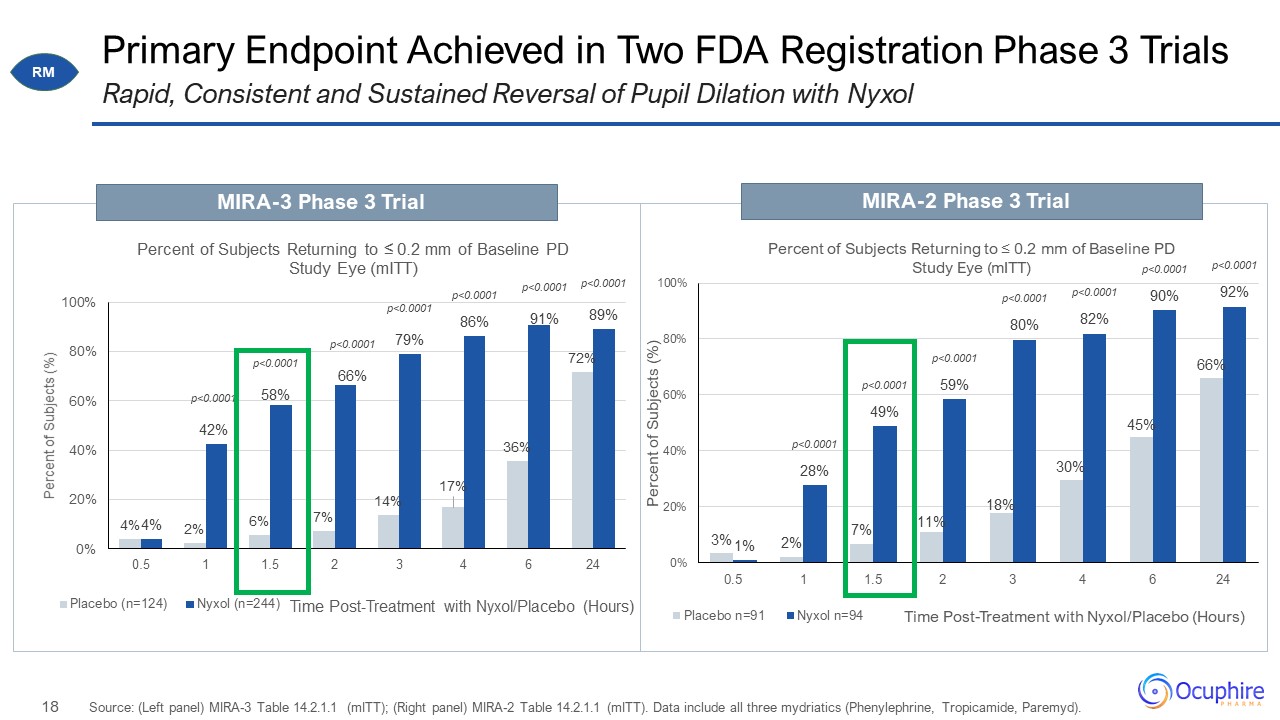
Primary Endpoint Achieved in Two FDA Registration Phase 3 Trials Source: (Left panel) MIRA-3 Table
14.2.1.1 (mITT); (Right panel) MIRA-2 Table 14.2.1.1 (mITT). Data include all three mydriatics (Phenylephrine, Tropicamide, Paremyd). Rapid, Consistent and Sustained Reversal of Pupil Dilation with Nyxol MIRA-3 Phase 3 Trial MIRA-2 Phase 3
Trial RM
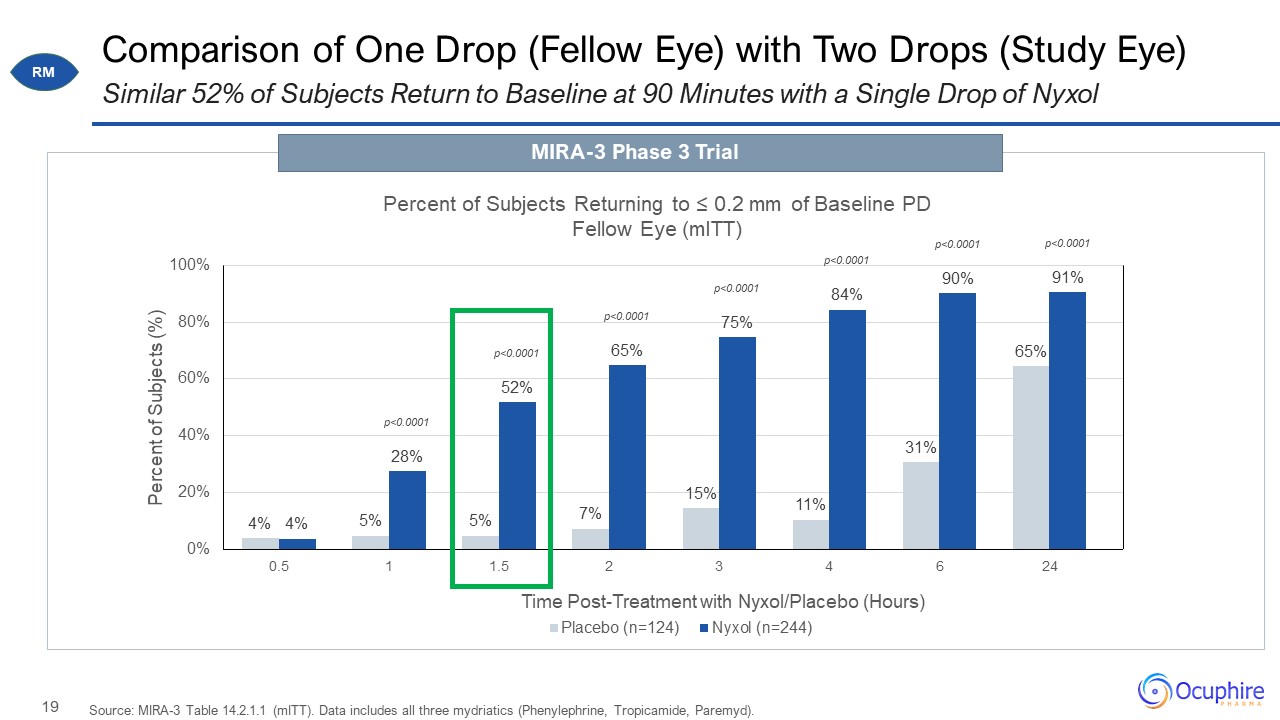
Comparison of One Drop (Fellow Eye) with Two Drops (Study Eye) Source: MIRA-3 Table 14.2.1.1 (mITT).
Data includes all three mydriatics (Phenylephrine, Tropicamide, Paremyd). Similar 52% of Subjects Return to Baseline at 90 Minutes with a Single Drop of Nyxol RM MIRA-3 Phase 3 Trial
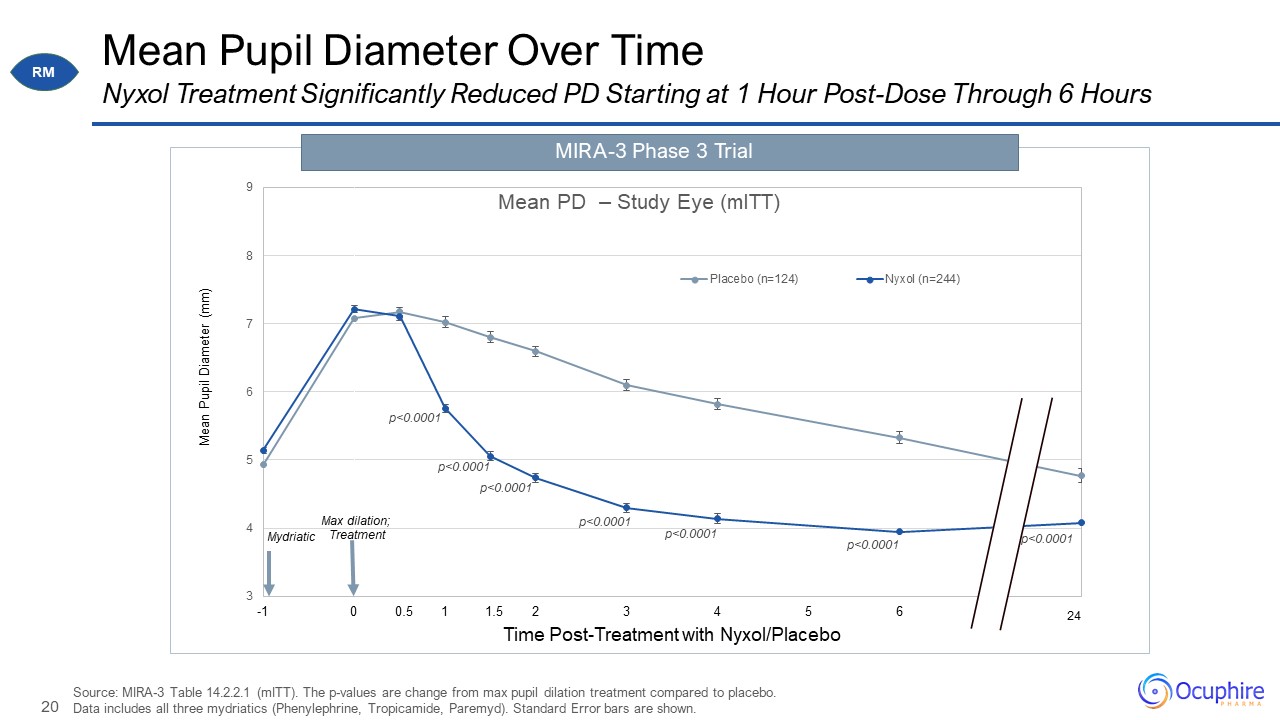
Mean Pupil Diameter Over Time Source: MIRA-3 Table 14.2.2.1 (mITT). The p-values are change from max
pupil dilation treatment compared to placebo. Data includes all three mydriatics (Phenylephrine, Tropicamide, Paremyd). Standard Error bars are shown. Nyxol Treatment Significantly Reduced PD Starting at 1 Hour Post-Dose Through 6
Hours MIRA-3 Phase 3 Trial 1.5 0.5 RM
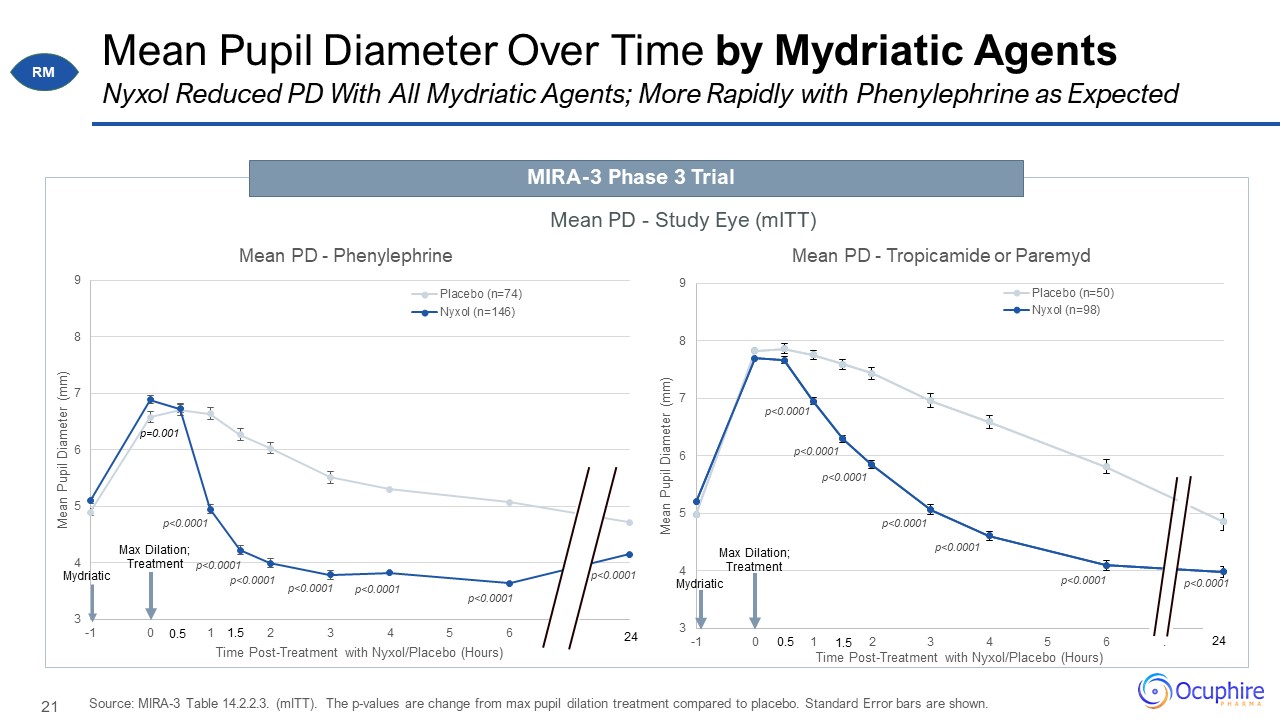
Mean Pupil Diameter Over Time by Mydriatic Agents Source: MIRA-3 Table 14.2.2.3. (mITT). The p-values
are change from max pupil dilation treatment compared to placebo. Standard Error bars are shown. Nyxol Reduced PD With All Mydriatic Agents; More Rapidly with Phenylephrine as Expected MIRA-3 Phase 3 Trial Mean PD - Study Eye
(mITT) RM p=0.001 Mydriatic Max Dilation; Treatment 1.5 1.5
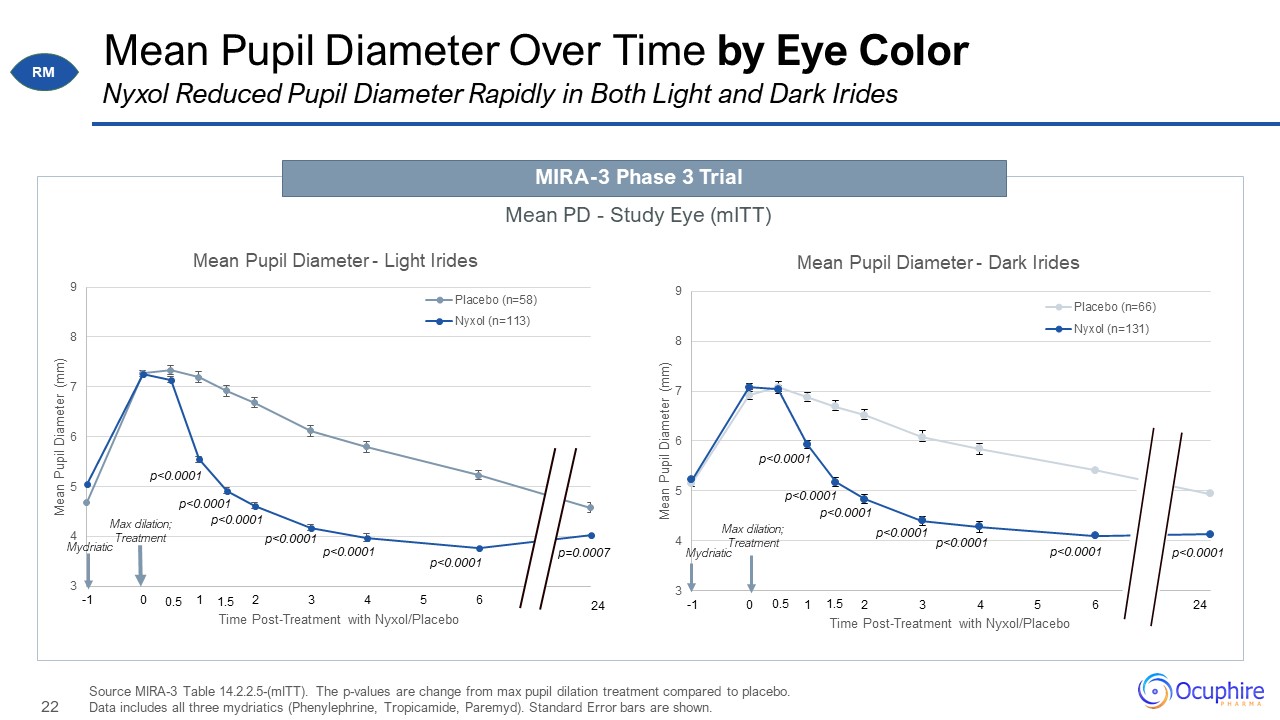
Mean Pupil Diameter Over Time by Eye Color Source MIRA-3 Table 14.2.2.5-(mITT). The p-values are
change from max pupil dilation treatment compared to placebo. Data includes all three mydriatics (Phenylephrine, Tropicamide, Paremyd). Standard Error bars are shown. Nyxol Reduced Pupil Diameter Rapidly in Both Light and Dark
Irides MIRA-3 Phase 3 Trial Mean PD - Study Eye (mITT) 24 0.5 0.5 1.5 1.5 RM Max dilation; Treatment Mydriatic p=0.0007 p<0.0001
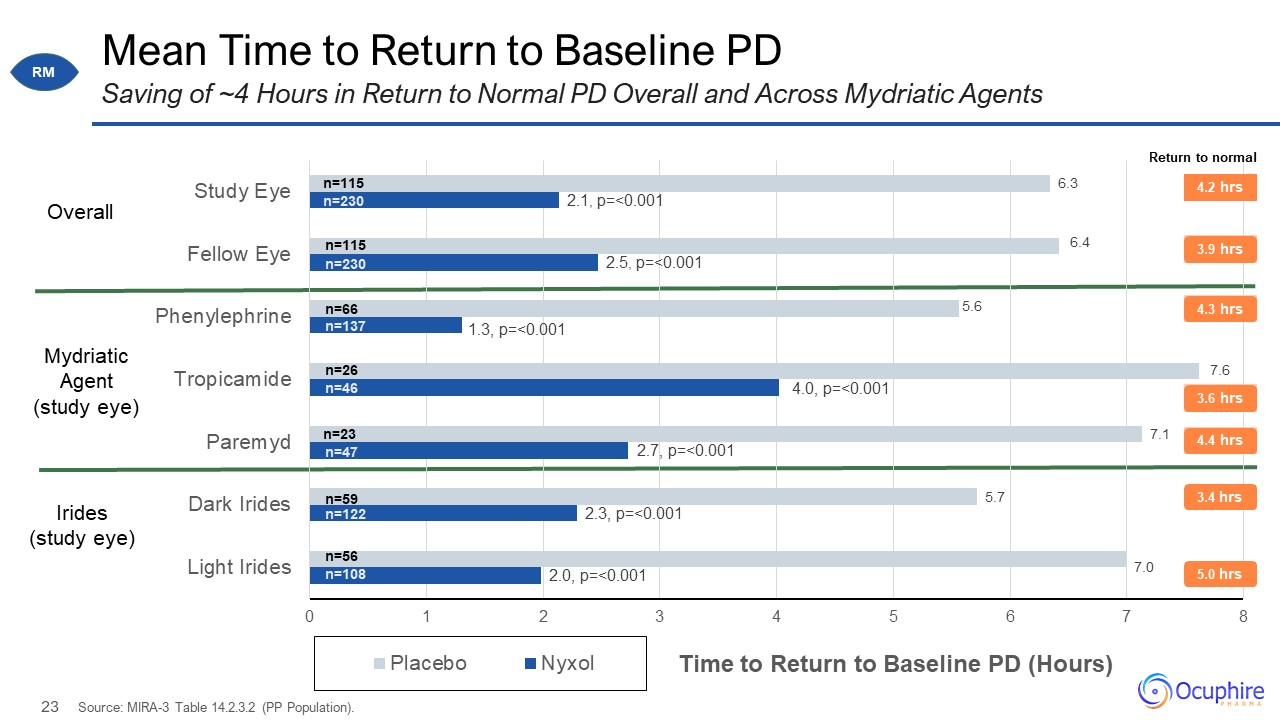
Mean Time to Return to Baseline PD Source: MIRA-3 Table 14.2.3.2 (PP Population). Saving of ~4 Hours
in Return to Normal PD Overall and Across Mydriatic Agents Overall Irides (study eye) Mydriatic Agent (study eye) n=230 n=115 n=230 n=115 n=66 n=137 n=26 n=46 n=23 n=47 n=59 n=122 n=56 n=108 RM 5.0 hrs 3.4 hrs
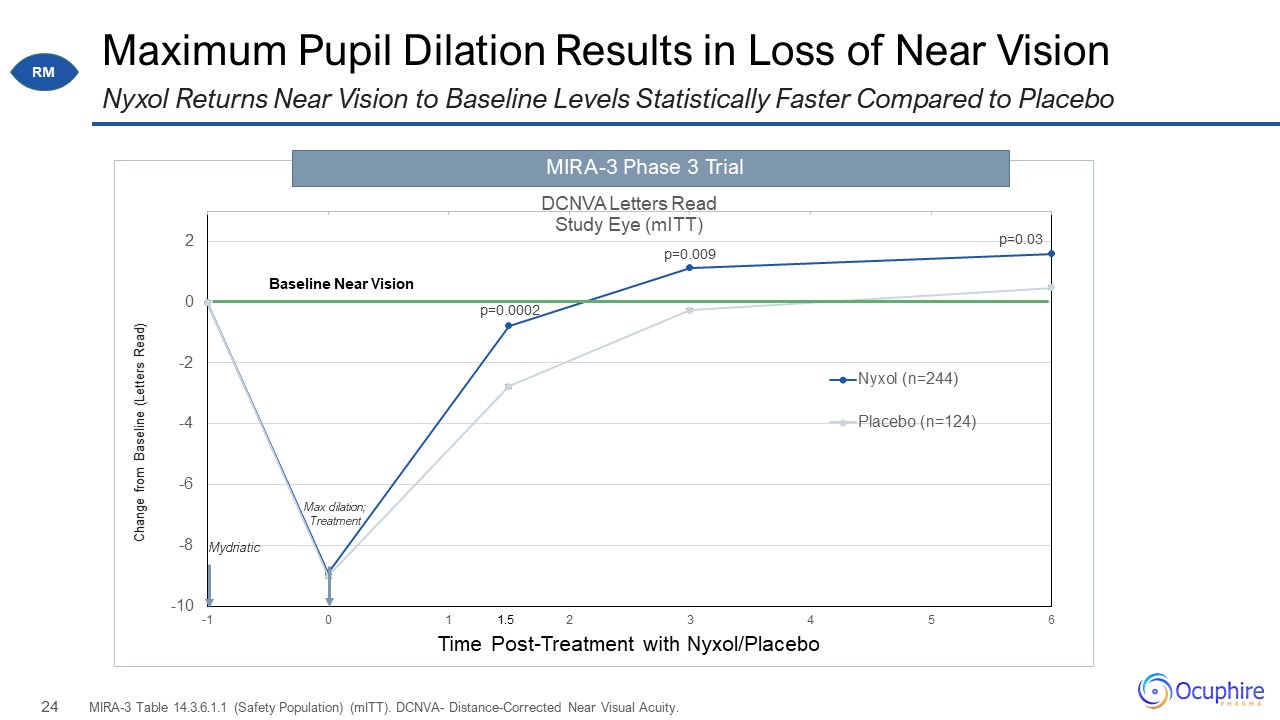
Maximum Pupil Dilation Results in Loss of Near Vision MIRA-3 Table 14.3.6.1.1 (Safety Population)
(mITT). DCNVA- Distance-Corrected Near Visual Acuity. Nyxol Returns Near Vision to Baseline Levels Statistically Faster Compared to Placebo RM Baseline Near Vision MIRA-3 Phase 3 Trial 1.5
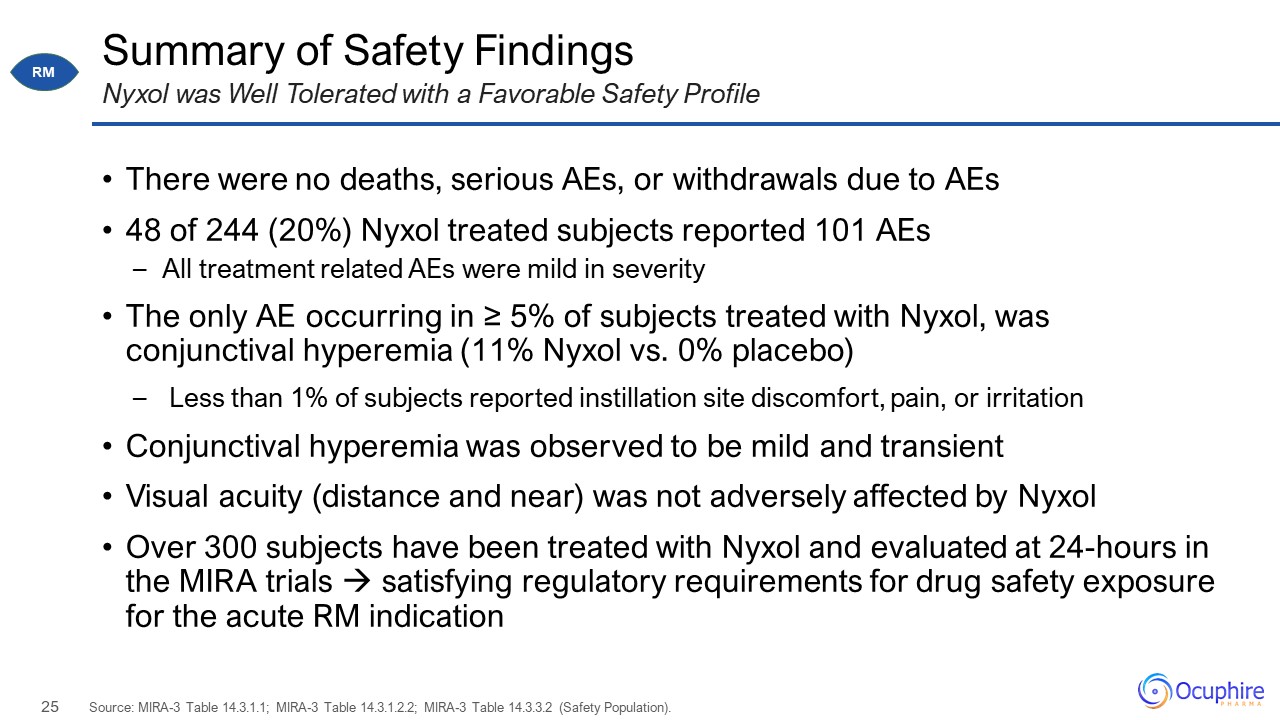
Summary of Safety Findings There were no deaths, serious AEs, or withdrawals due to AEs 48 of 244
(20%) Nyxol treated subjects reported 101 AEs All treatment related AEs were mild in severity The only AE occurring in ≥ 5% of subjects treated with Nyxol, was conjunctival hyperemia (11% Nyxol vs. 0% placebo) Less than 1% of subjects
reported instillation site discomfort, pain, or irritation Conjunctival hyperemia was observed to be mild and transient Visual acuity (distance and near) was not adversely affected by Nyxol Over 300 subjects have been treated with Nyxol
and evaluated at 24-hours in the MIRA trials satisfying regulatory requirements for drug safety exposure for the acute RM indication Source: MIRA-3 Table 14.3.1.1; MIRA-3 Table 14.3.1.2.2; MIRA-3 Table 14.3.3.2 (Safety Population). Nyxol
was Well Tolerated with a Favorable Safety Profile RM
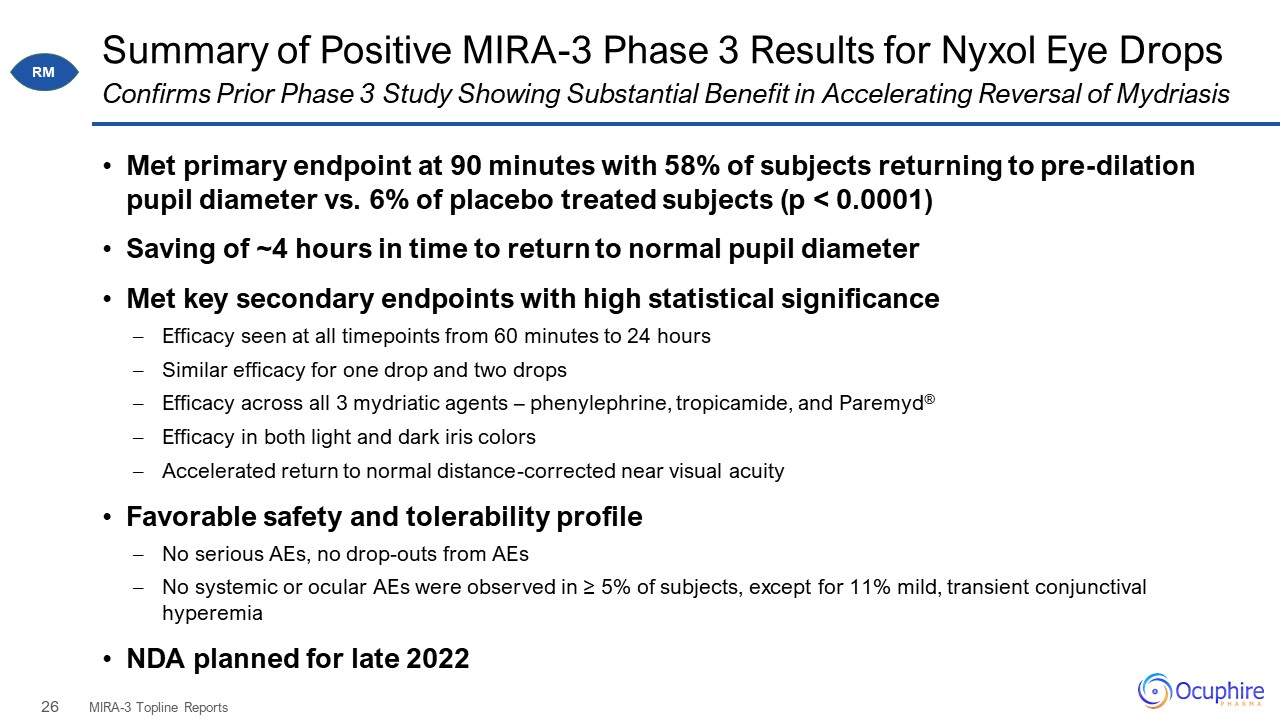
Summary of Positive MIRA-3 Phase 3 Results for Nyxol Eye Drops Met primary endpoint at 90 minutes with
58% of subjects returning to pre-dilation pupil diameter vs. 6% of placebo treated subjects (p < 0.0001) Saving of ~4 hours in time to return to normal pupil diameter Met key secondary endpoints with high statistical
significance Efficacy seen at all timepoints from 60 minutes to 24 hours Similar efficacy for one drop and two drops Efficacy across all 3 mydriatic agents – phenylephrine, tropicamide, and Paremyd® Efficacy in both light and dark iris
colors Accelerated return to normal distance-corrected near visual acuity Favorable safety and tolerability profile No serious AEs, no drop-outs from AEs No systemic or ocular AEs were observed in ≥ 5% of subjects, except for 11% mild,
transient conjunctival hyperemia NDA planned for late 2022 MIRA-3 Topline Reports Confirms Prior Phase 3 Study Showing Substantial Benefit in Accelerating Reversal of Mydriasis RM

Plans to NDA for Nyxol in RM
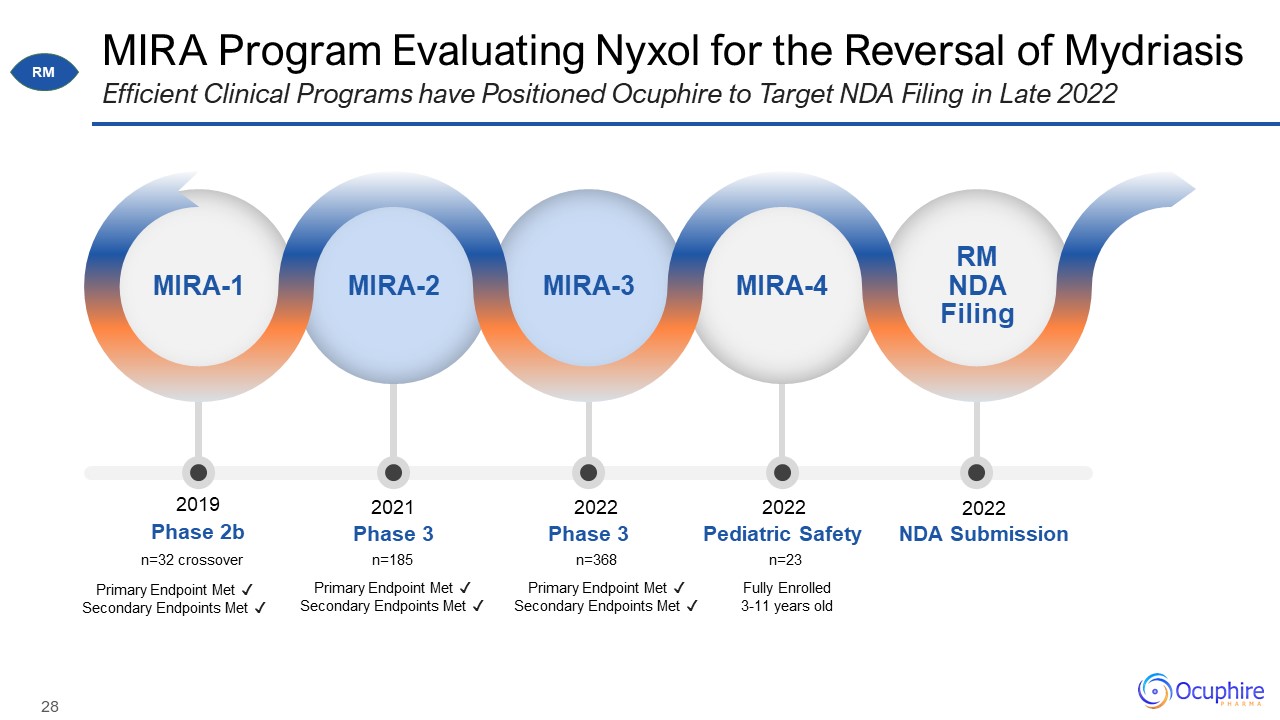
MIRA Program Evaluating Nyxol for the Reversal of Mydriasis Efficient Clinical Programs have
Positioned Ocuphire to Target NDA Filing in Late 2022 MIRA-1 MIRA-2 MIRA-3 MIRA-4 RM NDA Filing Phase 2b Phase 3 Phase 3 Pediatric Safety NDA Submission 2019 n=32 crossover n=185 n=368 n=23 2021 2022 2022 2022 Primary
Endpoint Met ✓ Secondary Endpoints Met ✓ Primary Endpoint Met ✓ Secondary Endpoints Met ✓ Primary Endpoint Met ✓ Secondary Endpoints Met ✓ RM Fully Enrolled 3-11 years old
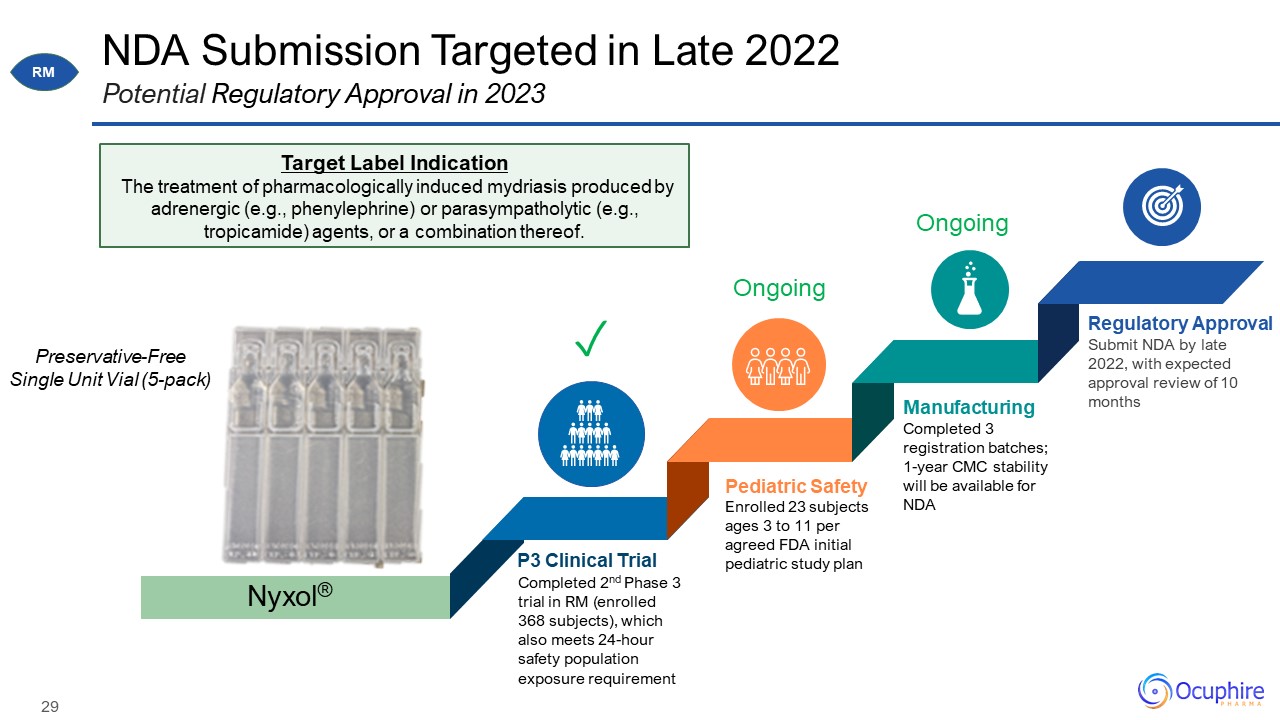
NDA Submission Targeted in Late 2022 Potential Regulatory Approval in 2023 Enrolled 23 subjects ages
3 to 11 per agreed FDA initial pediatric study plan Pediatric Safety Completed 2nd Phase 3 trial in RM (enrolled 368 subjects), which also meets 24-hour safety population exposure requirement P3 Clinical Trial Completed 3 registration
batches; 1-year CMC stability will be available for NDA Manufacturing Submit NDA by late 2022, with expected approval review of 10 months Regulatory Approval Nyxol® Target Label Indication The treatment of pharmacologically induced
mydriasis produced by adrenergic (e.g., phenylephrine) or parasympatholytic (e.g., tropicamide) agents, or a combination thereof. Preservative-Free Single Unit Vial (5-pack) ✓ Ongoing Ongoing RM

Reversal of Mydriasis Market Opportunity
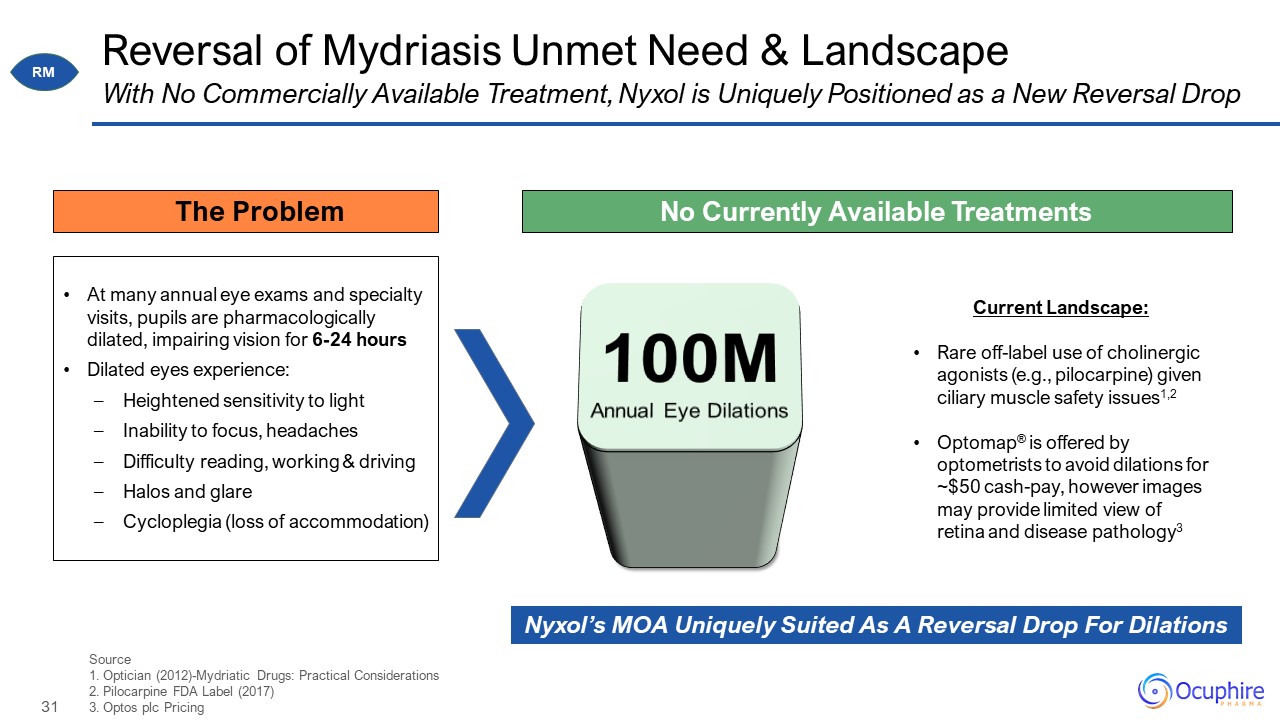
Reversal of Mydriasis Unmet Need & Landscape Source 1. Optician (2012)-Mydriatic Drugs: Practical
Considerations 2. Pilocarpine FDA Label (2017) 3. Optos plc Pricing With No Commercially Available Treatment, Nyxol is Uniquely Positioned as a New Reversal Drop At many annual eye exams and specialty visits, pupils are
pharmacologically dilated, impairing vision for 6-24 hours Dilated eyes experience: Heightened sensitivity to light Inability to focus, headaches Difficulty reading, working & driving Halos and glare Cycloplegia (loss of
accommodation) Current Landscape: Rare off-label use of cholinergic agonists (e.g., pilocarpine) given ciliary muscle safety issues1,2 Optomap® is offered by optometrists to avoid dilations for ~$50 cash-pay, however images may provide
limited view of retina and disease pathology3 No Currently Available Treatments 100M Annual Eye Dilations Nyxol’s MOA Uniquely Suited As A Reversal Drop For Dilations The Problem RM
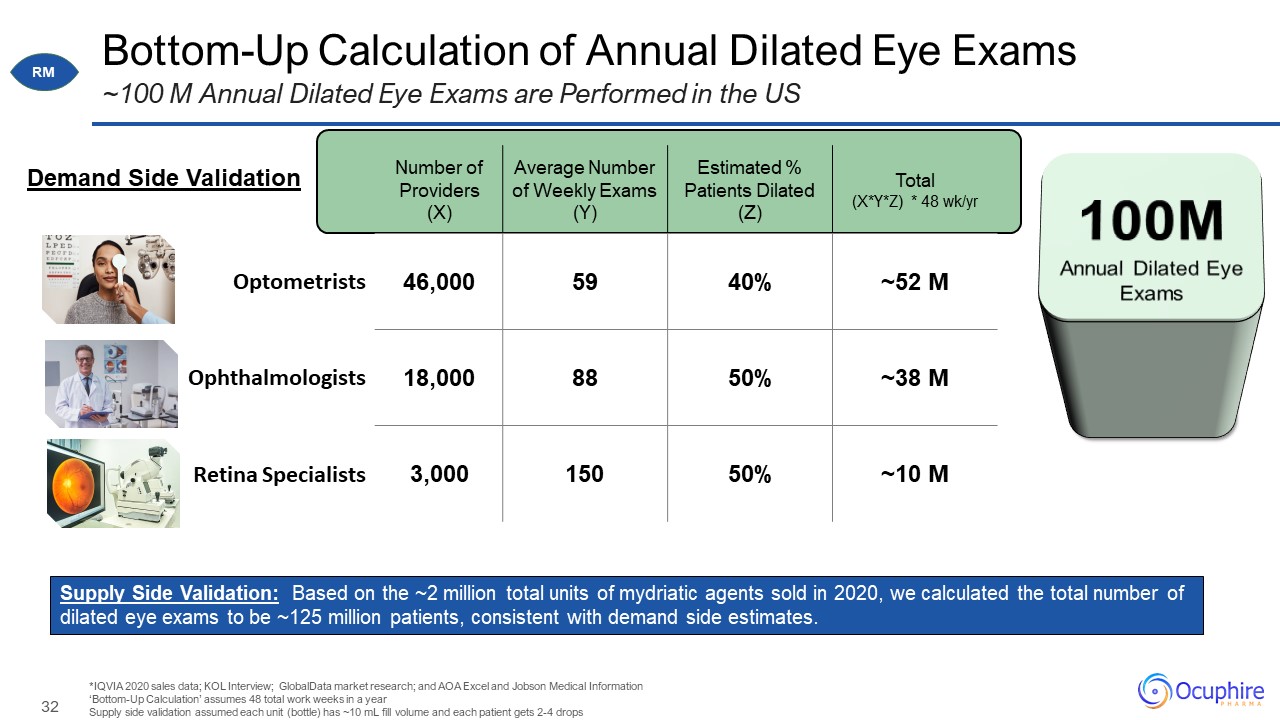
Bottom-Up Calculation of Annual Dilated Eye Exams ~100 M Annual Dilated Eye Exams are Performed in the
US *IQVIA 2020 sales data; KOL Interview; GlobalData market research; and AOA Excel and Jobson Medical Information ‘Bottom-Up Calculation’ assumes 48 total work weeks in a year Supply side validation assumed each unit (bottle) has ~10
mL fill volume and each patient gets 2-4 drops Number of Providers (X) Average Number of Weekly Exams (Y) Estimated % Patients Dilated(Z) Total(X*Y*Z) * 48 wk/yr Optometrists 46,000 59 40% ~52
M Ophthalmologists 18,000 88 50% ~38 M Retina Specialists 3,000 150 50% ~10 M 100M Annual Dilated Eye Exams Supply Side Validation: Based on the ~2 million total units of mydriatic agents sold in 2020, we calculated the total
number of dilated eye exams to be ~125 million patients, consistent with demand side estimates. Demand Side Validation RM
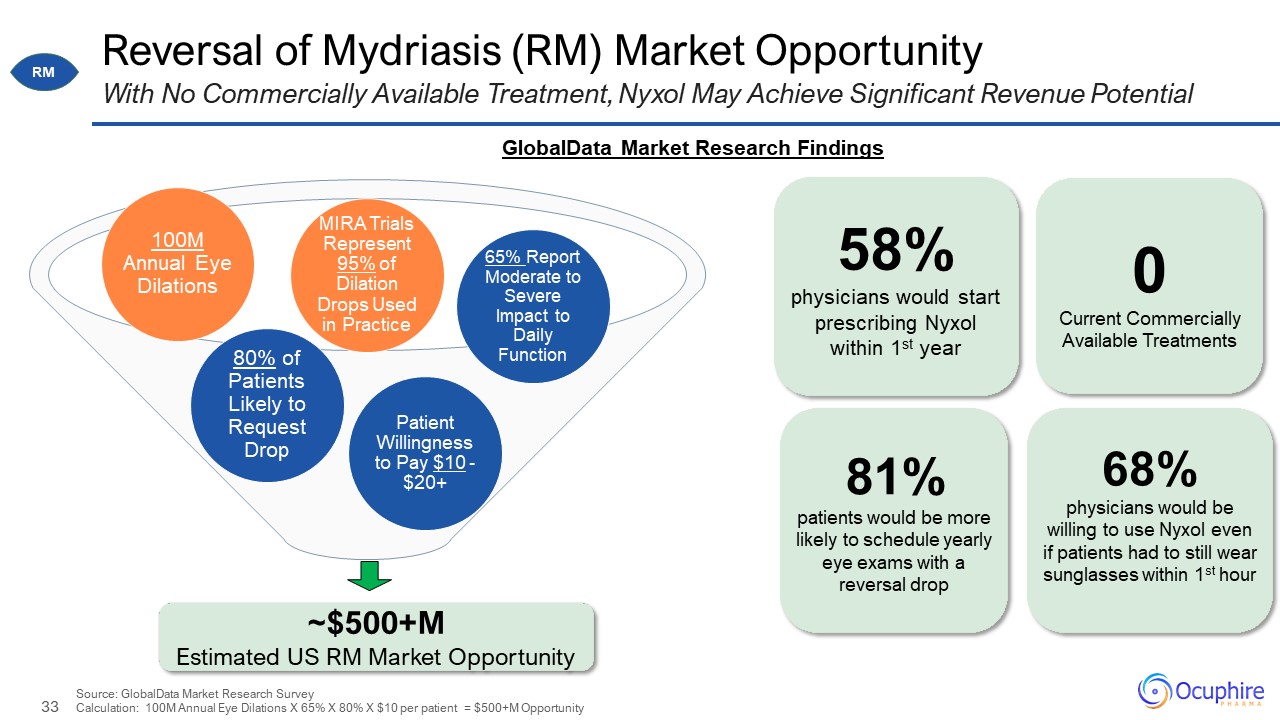
~$500+M Estimated US RM Market Opportunity Reversal of Mydriasis (RM) Market Opportunity With
No Commercially Available Treatment, Nyxol May Achieve Significant Revenue Potential Patient Willingness to Pay $10 - $20+ 65% Report Moderate to Severe Impact to Daily Function 100M Annual Eye Dilations 80% of Patients Likely to Request
Drop 58% physicians would start prescribing Nyxol within 1st year 81% patients would be more likely to schedule yearly eye exams with a reversal drop 0 Current Commercially Available Treatments MIRA Trials Represent 95% of Dilation
Drops Used in Practice Source: GlobalData Market Research SurveyCalculation: 100M Annual Eye Dilations X 65% X 80% X $10 per patient = $500+M Opportunity 68% physicians would be willing to use Nyxol even if patients had to still wear
sunglasses within 1st hour GlobalData Market Research Findings RM
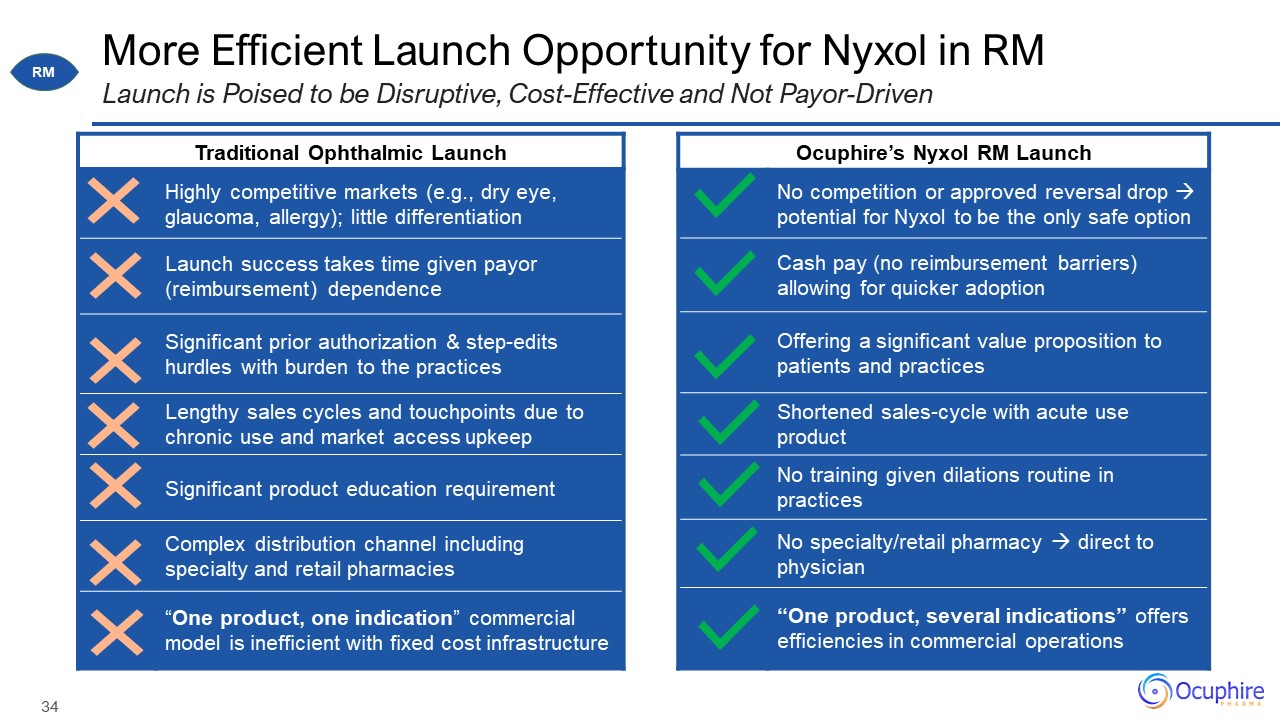
More Efficient Launch Opportunity for Nyxol in RM Launch is Poised to be Disruptive, Cost-Effective
and Not Payor-Driven Traditional Ophthalmic Launch Traditional Ophthalmic Launch Highly competitive markets (e.g., dry eye, glaucoma, allergy); little differentiation Launch success takes time given payor (reimbursement)
dependence Significant prior authorization & step-edits hurdles with burden to the practices Lengthy sales cycles and touchpoints due to chronic use and market access upkeep Significant product education requirement Complex
distribution channel including specialty and retail pharmacies “One product, one indication” commercial model is inefficient with fixed cost infrastructure Ocuphire’s Nyxol RM Launch Ocuphire’s Nyxol RM Launch No competition or approved
reversal drop potential for Nyxol to be the only safe option Cash pay (no reimbursement barriers) allowing for quicker adoption Offering a significant value proposition to patients and practices Shortened sales-cycle with acute use
product No training given dilations routine in practices No specialty/retail pharmacy direct to physician “One product, several indications” offers efficiencies in commercial operations RM
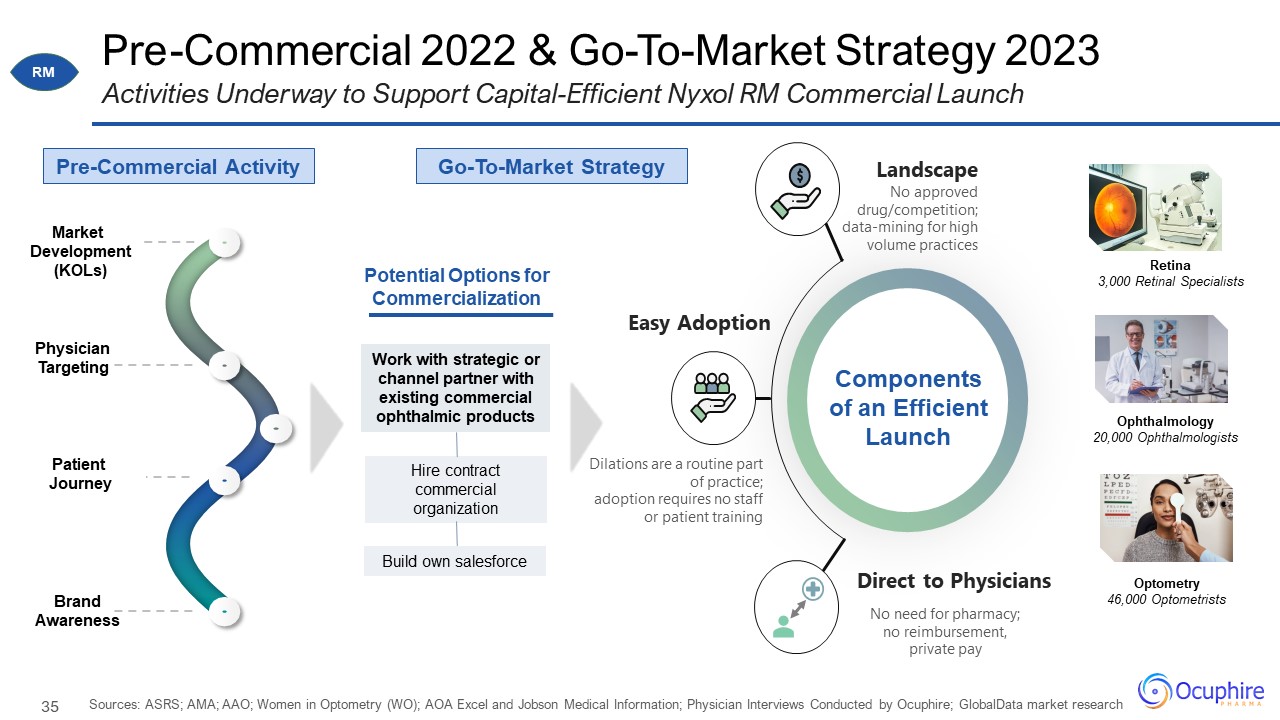
Pre-Commercial 2022 & Go-To-Market Strategy 2023 Sources: ASRS; AMA; AAO; Women in Optometry (WO);
AOA Excel and Jobson Medical Information; Physician Interviews Conducted by Ocuphire; GlobalData market research Activities Underway to Support Capital-Efficient Nyxol RM Commercial Launch Physician Targeting Pre-Commercial
Activity Market Development (KOLs) Patient Journey Brand Awareness No approved drug/competition; data-mining for high volume practices Landscape No need for pharmacy; no reimbursement, private pay Direct to Physicians Dilations
are a routine part of practice; adoption requires no staff or patient training Easy Adoption Components of an Efficient Launch Retina 3,000 Retinal Specialists Ophthalmology 20,000 Ophthalmologists Optometry 46,000 Optometrists
Potential Options for Commercialization Go-To-Market Strategy Work with strategic or channel partner with existing commercial ophthalmic products Hire contract commercial organization Build own salesforce RM

Upcoming Milestones
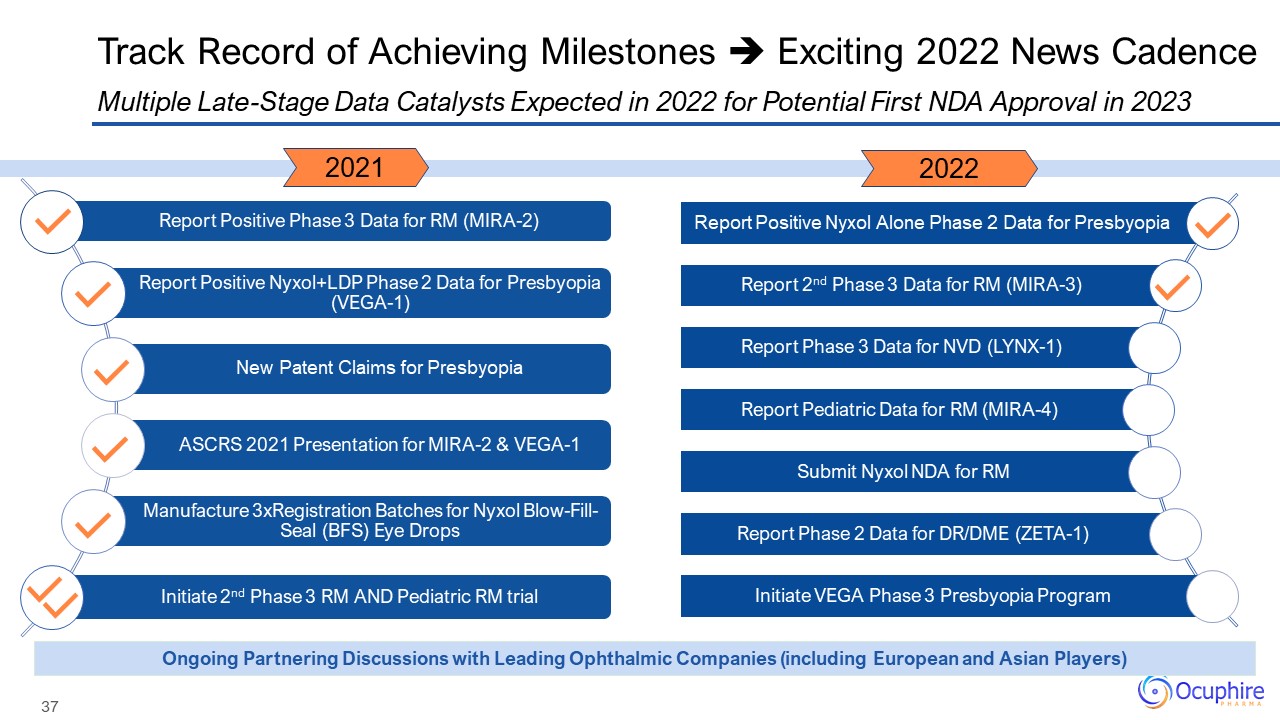
Track Record of Achieving Milestones Exciting 2022 News Cadence Ongoing Partnering Discussions with
Leading Ophthalmic Companies (including European and Asian Players) 2021 2022 Report Positive Nyxol Alone Phase 2 Data for Presbyopia Report 2nd Phase 3 Data for RM (MIRA-3) Report Phase 3 Data for NVD (LYNX-1) Report Pediatric Data
for RM (MIRA-4) Submit Nyxol NDA for RM Report Phase 2 Data for DR/DME (ZETA-1) Initiate VEGA Phase 3 Presbyopia Program Multiple Late-Stage Data Catalysts Expected in 2022 for Potential First NDA Approval in 2023