EXHIBIT 99.1
Published on October 14, 2022
Exhibit 99.1

Ocuphire KOL Event: APX3330 October 14, 2022

Disclosures and Forward-Looking Statements This presentation contains
“forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Such statements include, but are not limited to, statements concerning the success and timing of planned regulatory filings, including
planned NDA filings, pre-commercial activities, commercialization strategy and timelines, business strategy, product labels, cash runway, scalability, future clinical trials in reversal of mydriasis (RM), presbyopia (P), dim light/night
vision disturbance (NVD) and diabetic retinopathy (DR)/diabetic macular edema (DME), and the potential market opportunity in DR/DME. These forward-looking statements are based upon the Company’s current expectations and involve assumptions
that may never materialize or may prove to be incorrect. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, including,
without limitation: (i) the success, costs, and timing of regulatory submissions and preclinical and clinical trials, including enrollment and data readouts; (ii) regulatory requirements or developments; (iii) changes to clinical trial
designs and regulatory pathways; (iv) changes in capital resource requirements; (v) risks related to the inability of Ocuphire to obtain sufficient additional capital to continue to advance its product candidates and its preclinical programs;
(vi) legislative, regulatory, political, and economic developments, (vii) changes in market opportunities, (viii) the effects of COVID-19 on clinical programs and business operations, (ix) the success and timing of commercialization of any of
Ocuphire’s product candidates, including the scalability of Ocuphire’s product candidates, and (x) the maintenance of Ocuphire’s intellectual property rights. The foregoing review of important factors that could cause actual events to differ
from expectations should not be construed as exhaustive and should be read in conjunction with statements that are included herein and elsewhere, including the risk factors detailed in documents that have been and may be filed by the Company
from time to time with the SEC. All forward-looking statements contained in this presentation speak only as of the date on which they were made. The Company undertakes no obligation to update such statements to reflect events that occur or
circumstances that exist after the date on which they were made. The Company makes no representation or warranty, express or implied, as to the accuracy or completeness of the information contained in or incorporated by reference into this
presentation. Nothing contained in or incorporated by reference into this presentation is, or shall be relied upon as, a promise or representation by the Company as to the past or future. The Company assumes no responsibility for the
accuracy or completeness of any such information. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market shares and other data about our industry. This data involves a
number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. The trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use should not be
construed as an endorsement of such products.

Ocuphire APX3330 KOL Event: Agenda &
Speakers Speakers Speakers Agenda Time (EDT) Mina Sooch, MBA President & CEO and Founder Introductions & Company Overview 11:00 am – 11:10 am Caroline Baumal, MD Disease of Diabetic Retinopathy 11:10 am – 11:20 am Peter
Kaiser, MD Current DR/DME Treatment Landscape 11:20 am – 11:35 am David Lally, MD APX3330, Paradigm-Shifting Oral Treatment Option 11:35 am – 11:50 am Caroline Baumal, MD ZETA-1, Phase 2b Trial in Diabetic Retinopathy and Masked
Safety Data 11:50 am – 12:00 pm

Speakers Agenda Time (EDT) ZETA-1 Trial Design and Data Expectations 12:00
pm – 12:15 pm Q&A Closing Remarks Q&A Moderator: Corey Davis, PhD 12:15 pm – 12:30 pm Ocuphire APX3330 KOL Event: Agenda & Speakers Peter Kaiser, MD Caroline Baumal, MD David Lally, MD Mina Sooch, MBA Peter Kaiser,
MD Caroline Baumal, MD David Lally, MD President, CEO, and Founder Mitch Brigell, PhD Head, Clinical Strategy Mark Kelley, PhD APX Program Scientific Advisor

Company Overview Presenter: Mina Sooch, CEO and Founder of Ocuphire
Pharma Over 25 years of pharmaceutical and biotech experience as CEO, entrepreneur, venture capitalist, and strategy consultant Successful track record of hundreds of millions of capital raised for leading private/public biotech
companies Experience across multiple diseases (cardiovascular, oncology, renal, NASH, CNS, etc.) prior to ophthalmology Recipient of numerous awards, including Deal Makers of the Year in 2016 and Alumni Commencement Speaker WSU College of
Engineering in 2021 Mina Sooch, MBA Harvard University

Upcoming Catalysts in 4Q22: Topline Results APX3330 ZETA-1 P2b trial for
DR/DME NDA Filing for Nyxol for RM P = Presbyopia RM = Reversal of Mydriasis NVD = Night Vision Disturbances DR/DME = Diabetic Retinopathy/Diabetic Macular Edema Ocuphire PharmaNasdaq: OCUP Founded in 2018, Acquired 2 Lead Assets for
Front & Back of Eye Therapies with Novel MOAs & Patent Coverage to 2034+ Nyxol eyedrops Reversal of Mydriasis (“RM”) – eye dilation Presbyopia – age-related blurry near vision Night Vision Disturbance (“NVD”) – halos, glares,
starbursts APX3330 oral tablets Diabetic retinopathy (“DR”) – diabetes-related retinal (eye) disease Successful Execution of 5 Trials in last 2 Years with 6 Positive Phase 3 & Phase 2 Data Read-outs for Nyxol in RM, Presbyopia, and
NVD Potential 2023 commercialization opportunities in RM Near-term initiation planned for Presbyopia VEGA Phase 3 program with Nyxol alone and Nyxol with 0.4% Low Dose Pilocarpine as adjunctive therapy Four Large Markets (~$20B US total)
w/Unmet Needs and Limited to No Competition

Ocuphire Overview Two Late-Stage Clinical Assets Addressing Unmet Needs in
Multiple Large Markets Source: Eisai and Apexian Data; GlobalData Market Research Report, 2020; Company Estimates for US Market Size; Ocuphire internal estimates 11 Completed Phase 1 and Phase 2 Trials >340Subjects Dosed Patent
Coverage2034+ 12 Completed Phase 1,Phase 2, and Phase 3 Trials >650Subjects Dosed Exposure in Humans 28 Days Patent Coverage2034+ Exposure in Humans 365 Days Retina Refractive Presbyopia Reversal of Mydriasis Night
Vision Disturbances Diabetic Retinopathy Diabetic Macular Edema Phase 2b Last PatientLast VisitCompletedAug 22 ~128 M ~100 M ~2.4 M ~8 M 1st Phase 3 Positive Data Phase 2 Positive Data Single & Combo 2 Phase 3 Positive
Data & Ped P3 ~36 M APX3330 Oral REF-1 Inhibitor New Chemical Entity Phase 2b Data 4Q22 DevelopmentMilestone Prevalence (US) Nyxol Novel α1/ α2 Blocker 505(b)(2) NDA-Filing Ready DevelopmentMilestone Prevalence (US)

Track Record of Achieving Milestones Multiple Positive Data Readouts with
Multiple Catalysts Ahead Ongoing Partnering Discussions with Leading Ophthalmic Companies (including Europe and Asia) 2021 – 1H 2022 2H 2022 – 2023 Submit Nyxol NDA for RM Report APX3330 Phase 2b Data for DR/DME (ZETA-1) Initiate VEGA
Phase 3 Presbyopia Program Potential Nyxol Approval and Commercialization

Disease of Diabetic Retinopathy Presented by: Caroline Baumal, MD Professor
of Ophthalmology at Tufts Medical Center Co-Director of the Retina Service and Medical Retina Fellowship at New England Eye Center Authored over 170 publications, 33 book chapters on retinal diseases, and edited the book Treatment of
Diabetic Retinopathy Recognized by the American Society of Retinal Surgeons, The Retinal Hall of Fame and received such honors as the Donald J. Gass Beacon of Sight Award from the Florida Ophthalmologic Society and the ASRS Crystal Apple
award from the Vit-Buckle Society. Caroline Baumal, MD University of Toronto
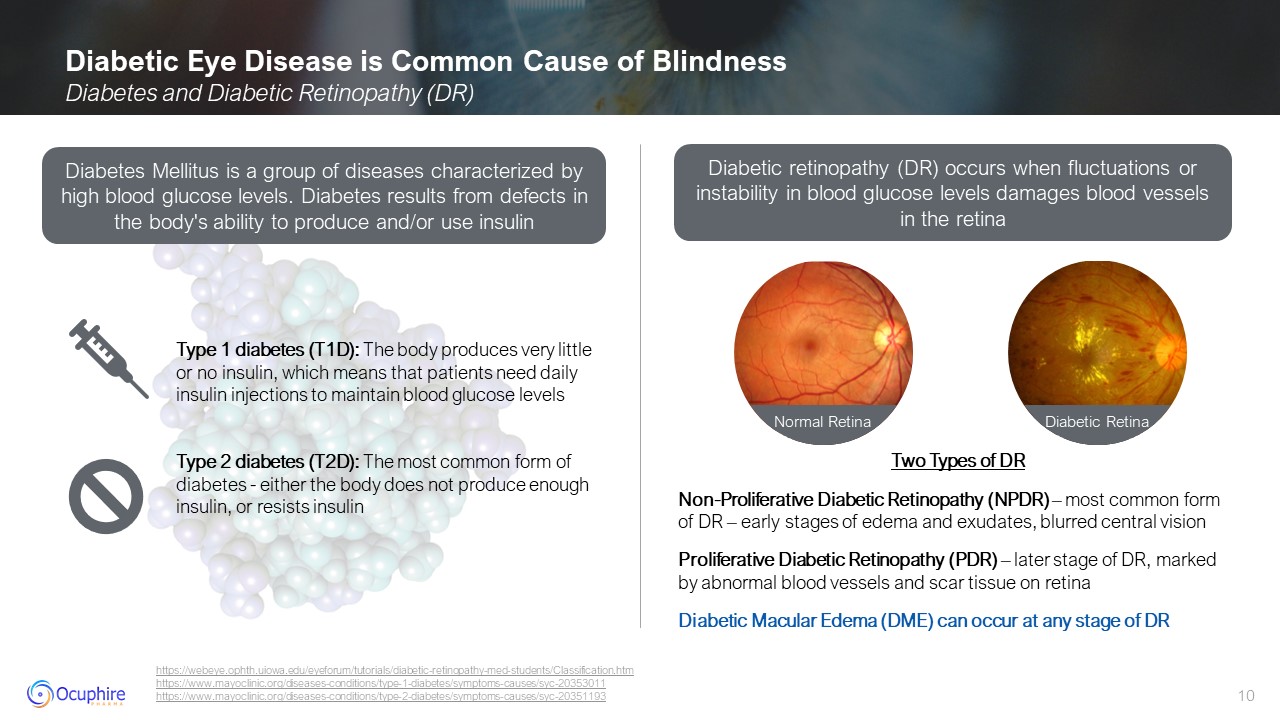
Diabetic Eye Disease is Common Cause of Blindness Diabetes and Diabetic
Retinopathy (DR) https://webeye.ophth.uiowa.edu/eyeforum/tutorials/diabetic-retinopathy-med-students/Classification.htmhttps://www.mayoclinic.org/diseases-conditions/type-1-diabetes/symptoms-causes/syc-20353011
https://www.mayoclinic.org/diseases-conditions/type-2-diabetes/symptoms-causes/syc-20351193 Two Types of DR Non-Proliferative Diabetic Retinopathy (NPDR) – most common form of DR – early stages of edema and exudates, blurred central
vision Proliferative Diabetic Retinopathy (PDR) – later stage of DR, marked by abnormal blood vessels and scar tissue on retina Diabetic Macular Edema (DME) can occur at any stage of DR Diabetes Mellitus is a group of diseases
characterized by high blood glucose levels. Diabetes results from defects in the body's ability to produce and/or use insulin Diabetic retinopathy (DR) occurs when fluctuations or instability in blood glucose levels damages blood vessels in
the retina Type 1 diabetes (T1D): The body produces very little or no insulin, which means that patients need daily insulin injections to maintain blood glucose levels Type 2 diabetes (T2D): The most common form of diabetes - either the
body does not produce enough insulin, or resists insulin Normal Retina Diabetic Retina
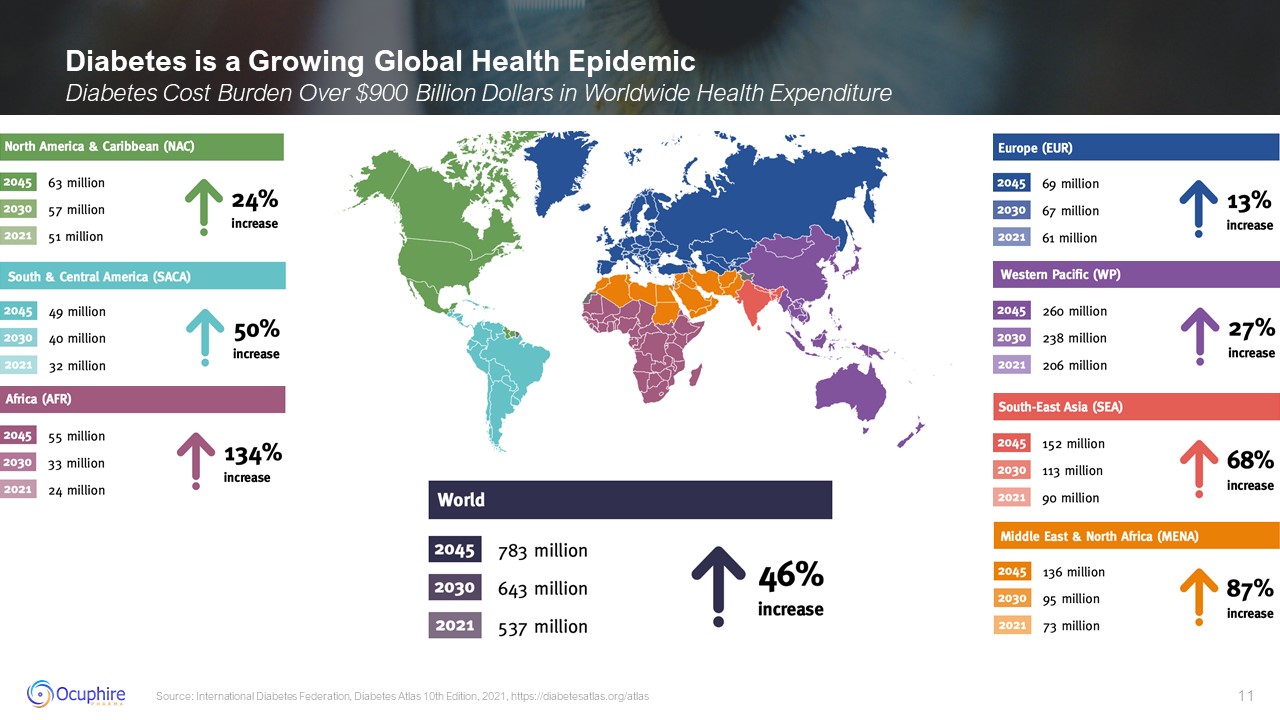
Diabetes is a Growing Global Health Epidemic Diabetes Cost Burden Over $900
Billion Dollars in Worldwide Health Expenditure Source: International Diabetes Federation, Diabetes Atlas 10th Edition, 2021, https://diabetesatlas.org/atlas
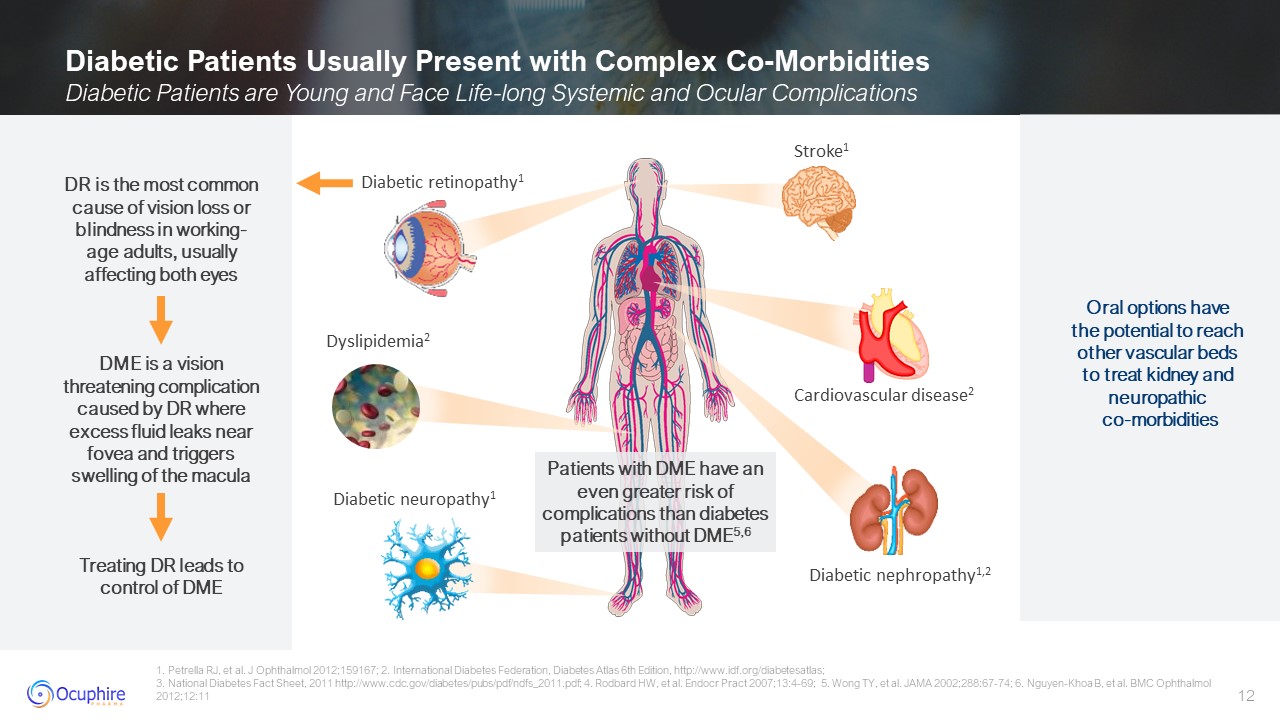
Diabetic Patients Usually Present with Complex Co-Morbidities Diabetic Patients
are Young and Face Life-long Systemic and Ocular Complications 1. Petrella RJ, et al. J Ophthalmol 2012;159167; 2. International Diabetes Federation, Diabetes Atlas 6th Edition, http://www.idf.org/diabetesatlas; 3. National Diabetes Fact
Sheet, 2011 http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf; 4. Rodbard HW, et al. Endocr Pract 2007;13:4-69; 5. Wong TY, et al. JAMA 2002;288:67-74; 6. Nguyen-Khoa B, et al. BMC Ophthalmol 2012;12:11 Patients with DME have an even
greater risk of complications than diabetes patients without DME5,6 DR is the most common cause of vision loss or blindness in working-age adults, usually affecting both eyes DME is a vision threatening complication caused by DR where
excess fluid leaks near fovea and triggers swelling of the macula Treating DR leads to control of DME Oral options have the potential to reach other vascular beds to treat kidney and neuropathic co-morbidities Diabetic
retinopathy1 Stroke1 Diabetic neuropathy1 Diabetic nephropathy1,2 Cardiovascular disease2 Dyslipidemia2
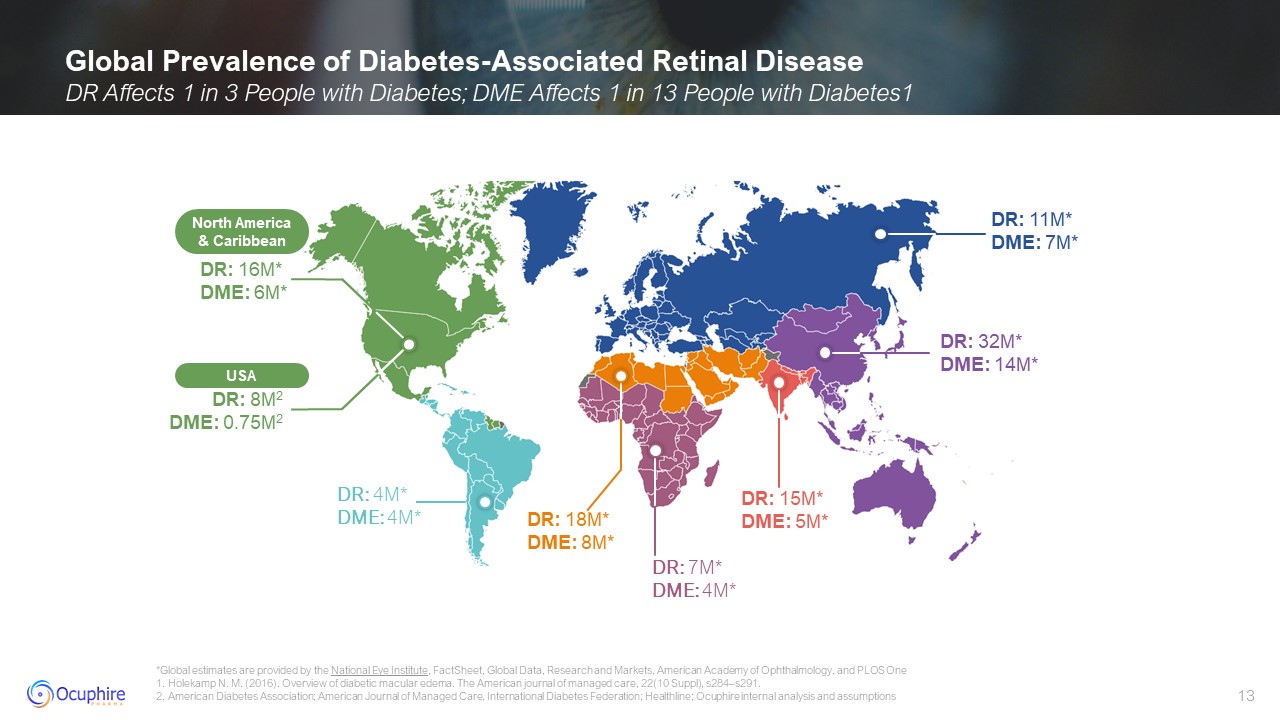
DR: 11M* DME: 7M* DR: 16M* DME: 6M* DR: 4M* DME: 4M* DR: 7M* DME:
4M* DR: 18M* DME: 8M* DR: 15M* DME: 5M* DR: 32M* DME: 14M* USA DR: 8M2 DME: 0.75M2 North America & Caribbean Global Prevalence of Diabetes-Associated Retinal Disease DR Affects 1 in 3 People with Diabetes; DME Affects 1 in 13
People with Diabetes1 *Global estimates are provided by the National Eye Institute, FactSheet, Global Data, Research and Markets, American Academy of Ophthalmology, and PLOS One 1. Holekamp N. M. (2016). Overview of diabetic macular
edema. The American journal of managed care, 22(10 Suppl), s284–s291. 2. American Diabetes Association; American Journal of Managed Care, International Diabetes Federation; Healthline; Ocuphire internal analysis and assumptions
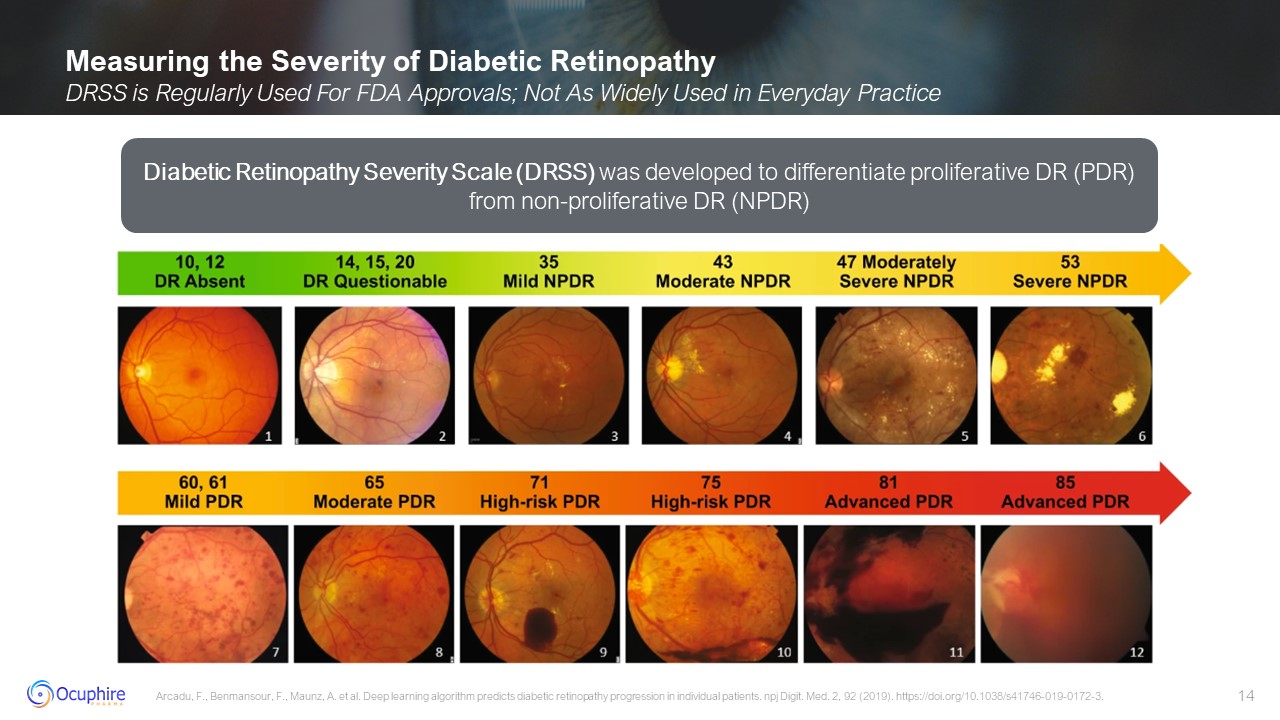
Measuring the Severity of Diabetic Retinopathy DRSS is Regularly Used For FDA
Approvals; Not As Widely Used in Everyday Practice Arcadu, F., Benmansour, F., Maunz, A. et al. Deep learning algorithm predicts diabetic retinopathy progression in individual patients. npj Digit. Med. 2, 92 (2019).
https://doi.org/10.1038/s41746-019-0172-3. Diabetic Retinopathy Severity Scale (DRSS) was developed to differentiate proliferative DR (PDR) from non-proliferative DR (NPDR)
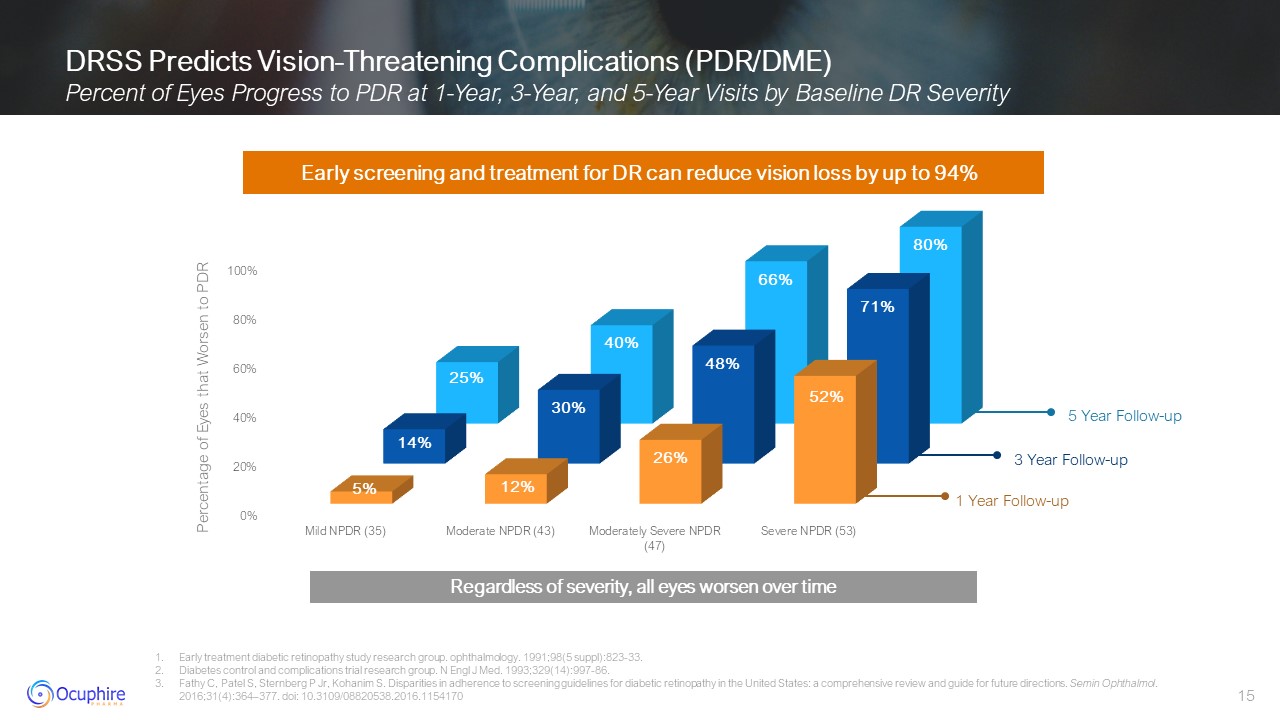
DRSS Predicts Vision-Threatening Complications (PDR/DME) Percent of Eyes
Progress to PDR at 1-Year, 3-Year, and 5-Year Visits by Baseline DR Severity Early treatment diabetic retinopathy study research group. ophthalmology. 1991;98(5 suppl):823-33. Diabetes control and complications trial research group. N
Engl J Med. 1993;329(14):997-86. Fathy C, Patel S, Sternberg P Jr, Kohanim S. Disparities in adherence to screening guidelines for diabetic retinopathy in the United States: a comprehensive review and guide for future directions. Semin
Ophthalmol. 2016;31(4):364–377. doi: 10.3109/08820538.2016.1154170 Early screening and treatment for DR can reduce vision loss by up to 94% 1 Year Follow-up 3 Year Follow-up 5 Year Follow-up Percentage of Eyes that Worsen to
PDR Regardless of severity, all eyes worsen over time
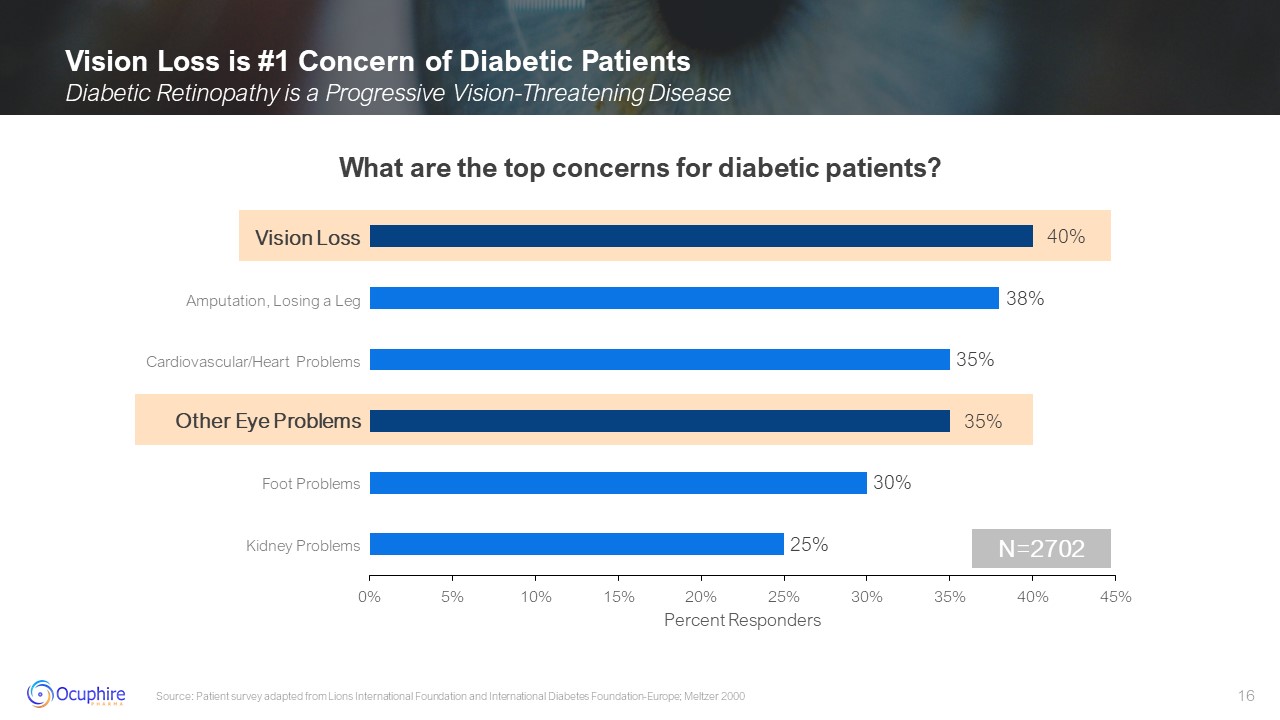
Vision Loss Other Eye Problems Amputation, Losing a Leg Cardiovascular/Heart
Problems Foot Problems Kidney Problems Vision Loss is #1 Concern of Diabetic Patients Diabetic Retinopathy is a Progressive Vision-Threatening Disease Source: Patient survey adapted from Lions International Foundation and International
Diabetes Foundation-Europe; Meltzer 2000 N=2702 What are the top concerns for diabetic patients?

Early Management of Diabetic Retinopathy Poor Adherence to Medical Management
and Lifestyle Options Worsen DR Source: Zhang X et al. Cell Biosci. 2014;14:4:27 Control of Blood Sugar Control of Blood Pressure Smoking Cessation Control of Lipids Medical and lifestyle management is first line of treatment
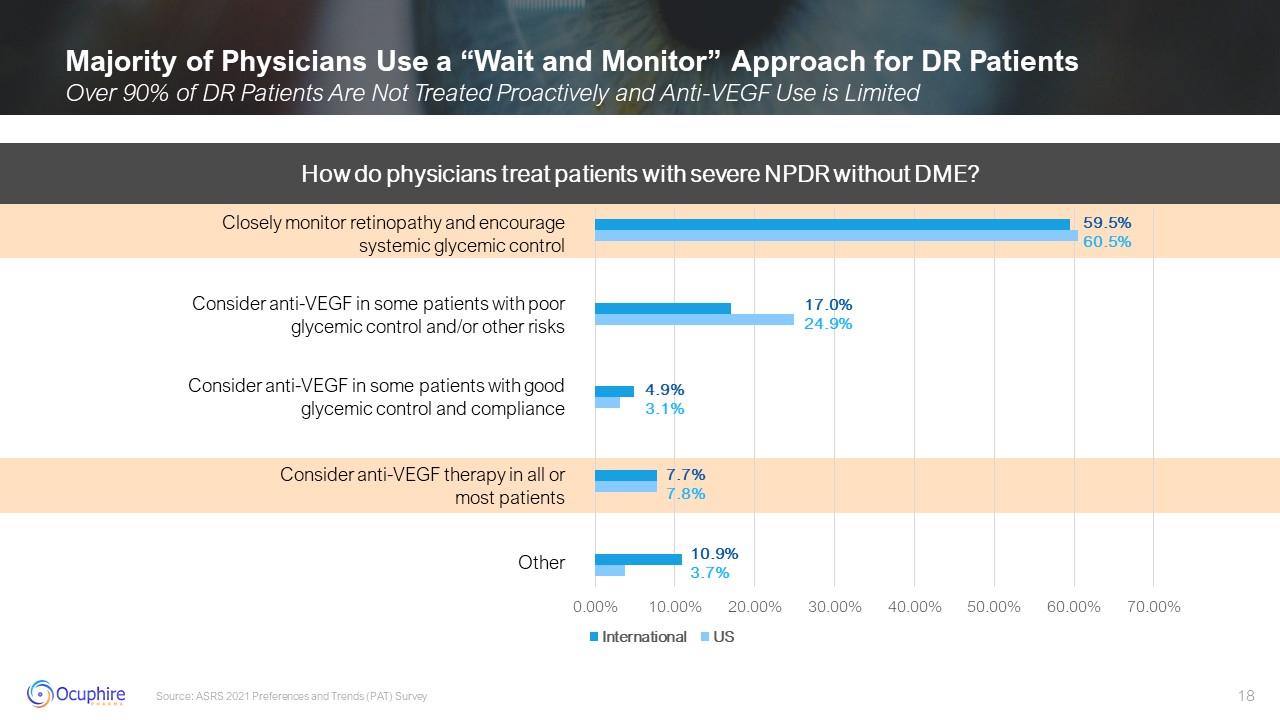
Majority of Physicians Use a “Wait and Monitor” Approach for DR Patients Over
90% of DR Patients Are Not Treated Proactively and Anti-VEGF Use is Limited Source: ASRS 2021 Preferences and Trends (PAT) Survey How do physicians treat patients with severe NPDR without DME? Closely monitor retinopathy and encourage
systemic glycemic control Consider anti-VEGF in some patients with poor glycemic control and/or other risks Consider anti-VEGF in some patients with good glycemic control and compliance Consider anti-VEGF therapy in all or most
patients Other
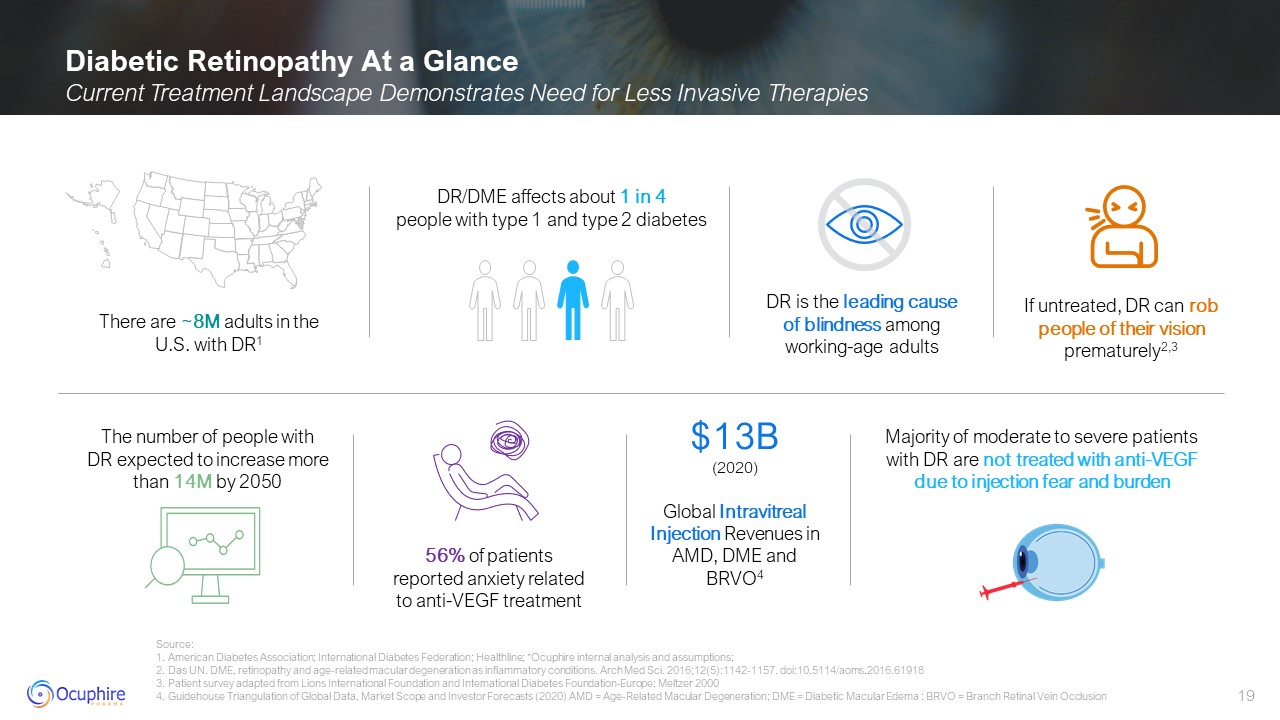
Diabetic Retinopathy At a Glance Current Treatment Landscape Demonstrates Need
for Less Invasive Therapies Source:1. American Diabetes Association; International Diabetes Federation; Healthline; *Ocuphire internal analysis and assumptions; 2. Das UN. DME, retinopathy and age-related macular degeneration as
inflammatory conditions. Arch Med Sci. 2016;12(5):1142-1157. doi:10.5114/aoms.2016.61918 3. Patient survey adapted from Lions International Foundation and International Diabetes Foundation-Europe; Meltzer 2000 4. Guidehouse Triangulation of
Global Data, Market Scope and Investor Forecasts (2020) AMD = Age-Related Macular Degeneration; DME = Diabetic Macular Edema ; BRVO = Branch Retinal Vein Occlusion The number of people with DR expected to increase more than 14M by
2050 There are ~8M adults in the U.S. with DR1 DR is the leading cause of blindness among working-age adults If untreated, DR can rob people of their vision prematurely2,3 56% of patients reported anxiety related to anti-VEGF
treatment DR/DME affects about 1 in 4 people with type 1 and type 2 diabetes Majority of moderate to severe patients with DR are not treated with anti-VEGF due to injection fear and burden $13B (2020) Global Intravitreal Injection
Revenues in AMD, DME and BRVO4
Current DR/DME Treatment Landscape Presented by: Peter Kaiser, MD Chaney
Family Endowed Chair in Ophthalmology Research, Professor of Ophthalmology, Cleveland Clinic Lerner College of Medicine and Cole Eye Institute Clinical research expert, serving as a Study Chairman of 5 major, multi-center, international
trials, and principal investigator for numerous studies for AMD, DR, and other retinal disorders. Major contributions to medical literature having authored 7 textbooks, more than 250 peer-reviewed papers Recognized by American Academy of
Ophthalmology and American Society of Retina Specialist with Senior Achievement Awards. Peter Kaiser, MD Harvard Medical School
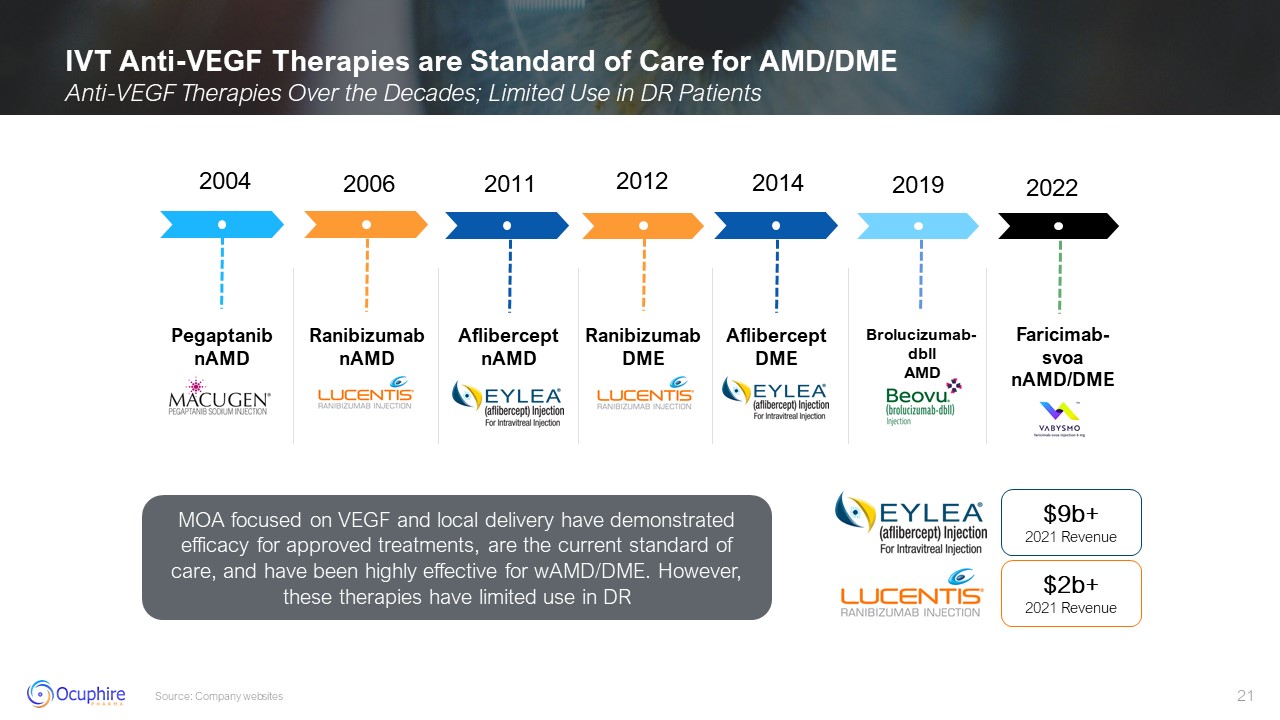
2004 2006 2011 2012 Ranibizumab nAMD 2014 2019 Aflibercept DME 2022 MOA
focused on VEGF and local delivery have demonstrated efficacy for approved treatments, are the current standard of care, and have been highly effective for wAMD/DME. However, these therapies have limited use in
DR AfliberceptnAMD Faricimab-svoa nAMD/DME Pegaptanib nAMD $9b+ 2021 Revenue $2b+ 2021 Revenue Ranibizumab DME Brolucizumab-dbll AMD IVT Anti-VEGF Therapies are Standard of Care for AMD/DME Anti-VEGF Therapies Over the Decades;
Limited Use in DR Patients Source: Company websites
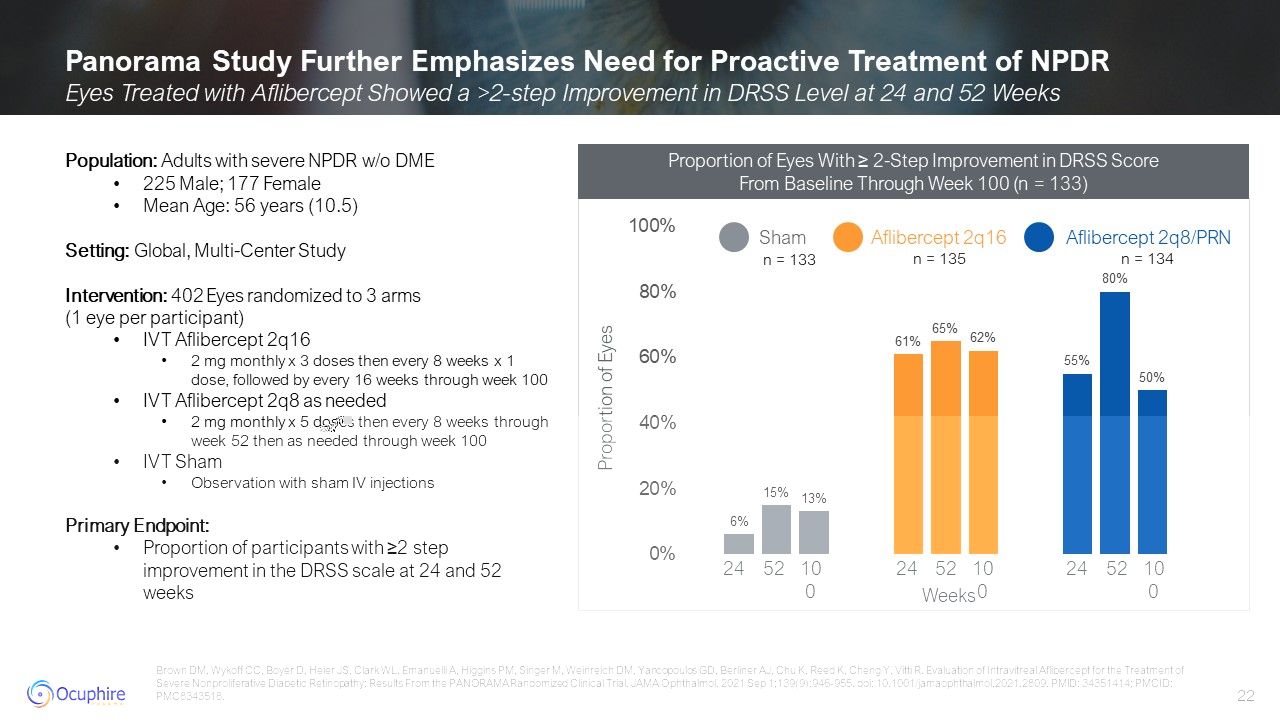
Panorama Study Further Emphasizes Need for Proactive Treatment of NPDR Eyes
Treated with Aflibercept Showed a >2-step Improvement in DRSS Level at 24 and 52 Weeks Brown DM, Wykoff CC, Boyer D, Heier JS, Clark WL, Emanuelli A, Higgins PM, Singer M, Weinreich DM, Yancopoulos GD, Berliner AJ, Chu K, Reed K, Cheng Y,
Vitti R. Evaluation of Intravitreal Aflibercept for the Treatment of Severe Nonproliferative Diabetic Retinopathy: Results From the PANORAMA Randomized Clinical Trial. JAMA Ophthalmol. 2021 Sep 1;139(9):946-955. doi:
10.1001/jamaophthalmol.2021.2809. PMID: 34351414; PMCID: PMC8343518. Population: Adults with severe NPDR w/o DME 225 Male; 177 Female Mean Age: 56 years (10.5) Setting: Global, Multi-Center Study Intervention: 402 Eyes randomized to 3
arms (1 eye per participant) IVT Aflibercept 2q16 2 mg monthly x 3 doses then every 8 weeks x 1 dose, followed by every 16 weeks through week 100 IVT Aflibercept 2q8 as needed 2 mg monthly x 5 doses then every 8 weeks through week 52
then as needed through week 100 IVT Sham Observation with sham IV injections Primary Endpoint: Proportion of participants with ≥2 step improvement in the DRSS scale at 24 and 52
weeks 24 52 100 24 52 100 24 52 100 Weeks Proportion of Eyes Aflibercept 2q8/PRN n = 133 n = 135 n = 134 Proportion of Eyes With ≥ 2-Step Improvement in DRSS Score From Baseline Through Week 100 (n = 133)
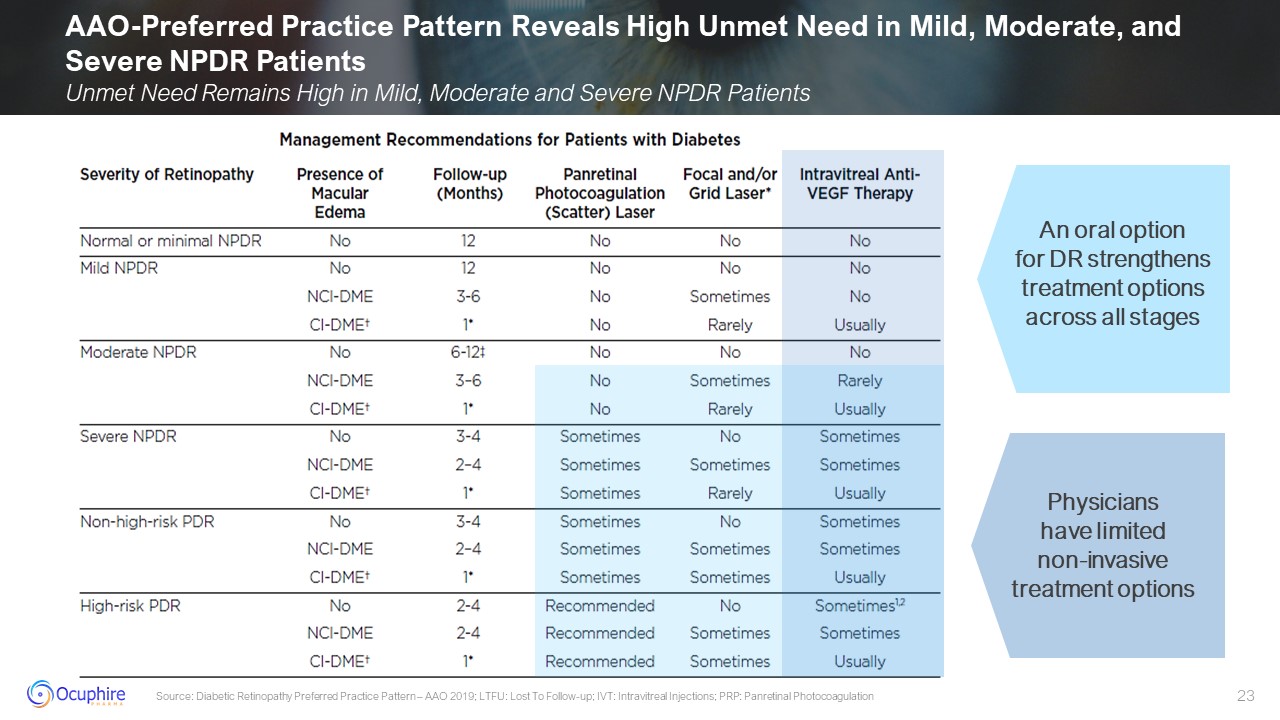
AAO-Preferred Practice Pattern Reveals High Unmet Need in Mild, Moderate, and
Severe NPDR Patients Unmet Need Remains High in Mild, Moderate and Severe NPDR Patients Source: Diabetic Retinopathy Preferred Practice Pattern – AAO 2019; LTFU: Lost To Follow-up; IVT: Intravitreal Injections; PRP: Panretinal
Photocoagulation Physicians have limited non-invasive treatment options An oral option for DR strengthens treatment options across all stages
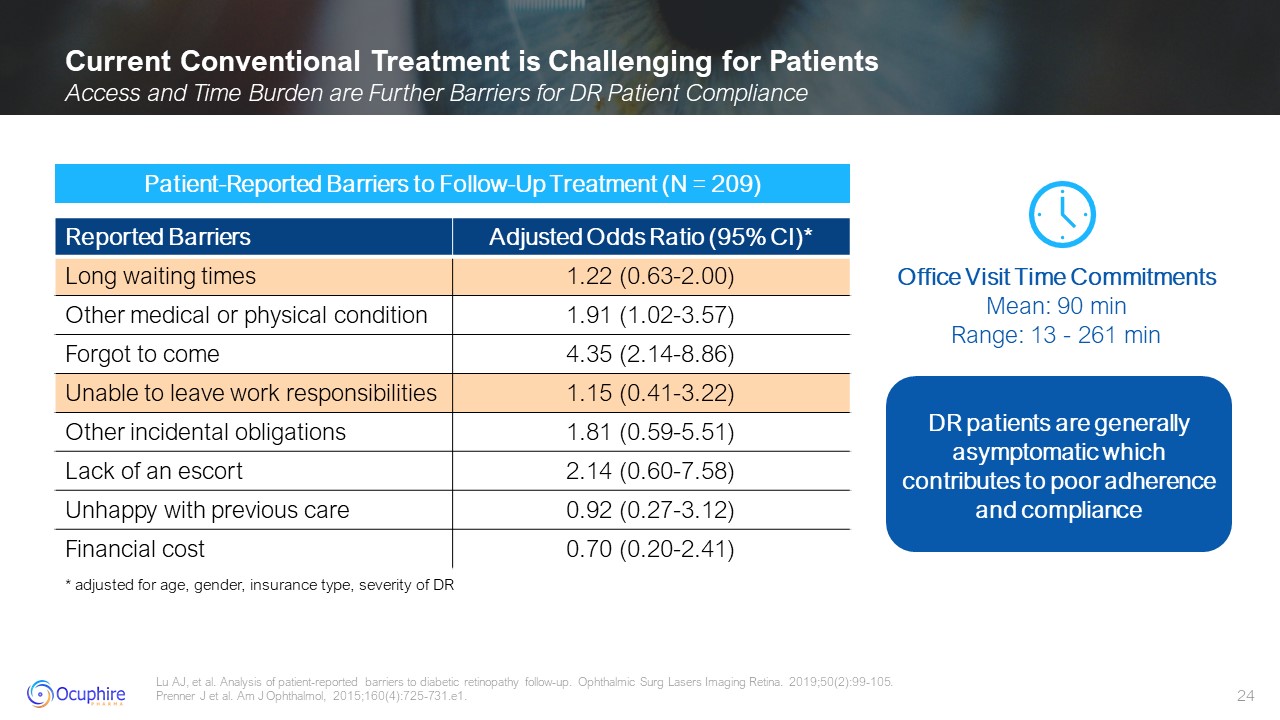
Current Conventional Treatment is Challenging for Patients Access and Time
Burden are Further Barriers for DR Patient Compliance Lu AJ, et al. Analysis of patient-reported barriers to diabetic retinopathy follow-up. Ophthalmic Surg Lasers Imaging Retina. 2019;50(2):99-105. Prenner J et al. Am J Ophthalmol,
2015;160(4):725-731.e1. Patient-Reported Barriers to Follow-Up Treatment (N = 209) Office Visit Time Commitments Mean: 90 min Range: 13 - 261 min * adjusted for age, gender, insurance type, severity of DR DR patients are generally
asymptomatic which contributes to poor adherence and compliance Reported Barriers Adjusted Odds Ratio (95% CI)* Long waiting times 1.22 (0.63-2.00) Other medical or physical condition 1.91 (1.02-3.57) Forgot to come 4.35
(2.14-8.86) Unable to leave work responsibilities 1.15 (0.41-3.22) Other incidental obligations 1.81 (0.59-5.51) Lack of an escort 2.14 (0.60-7.58) Unhappy with previous care 0.92 (0.27-3.12) Financial cost 0.70 (0.20-2.41)
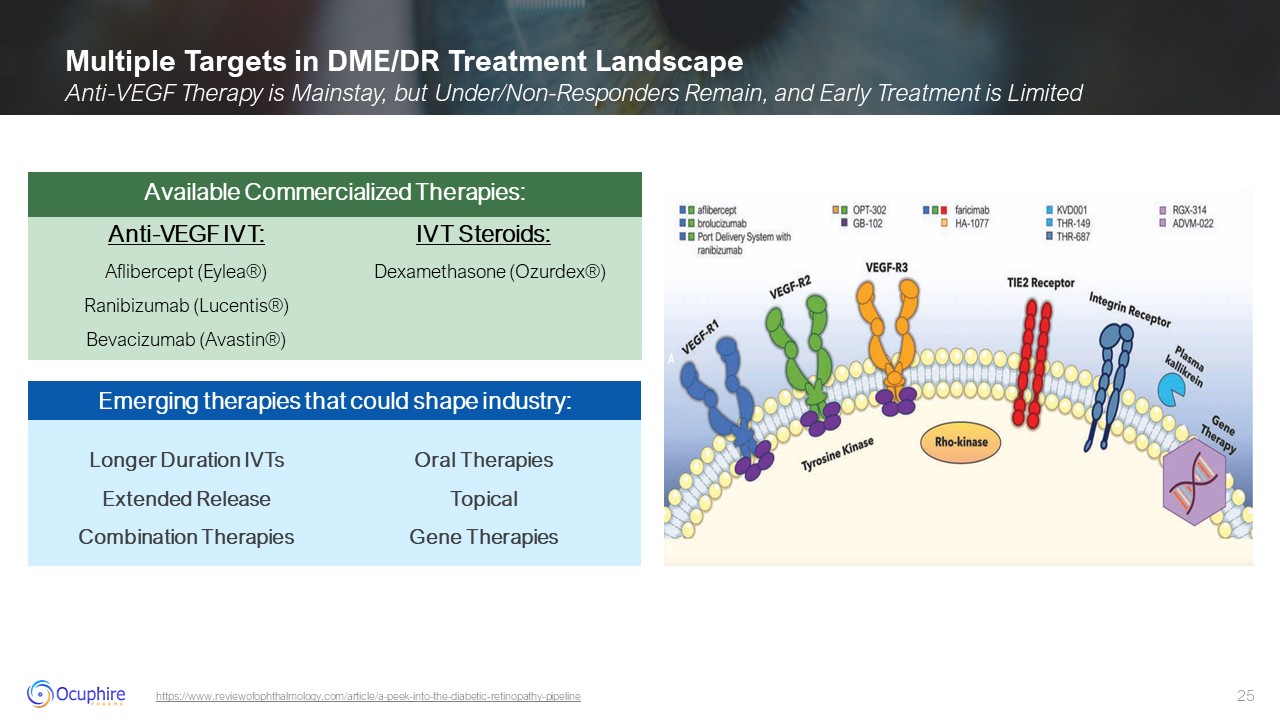
Multiple Targets in DME/DR Treatment Landscape Anti-VEGF Therapy is Mainstay,
but Under/Non-Responders Remain, and Early Treatment is Limited https://www.reviewofophthalmology.com/article/a-peek-into-the-diabetic-retinopathy-pipeline Longer Duration IVTs Extended Release Combination Therapies Oral
Therapies Topical Gene Therapies Emerging therapies that could shape industry: Anti-VEGF IVT: Aflibercept (Eylea®) Ranibizumab (Lucentis®) Bevacizumab (Avastin®) IVT Steroids: Dexamethasone (Ozurdex®) Available Commercialized
Therapies:
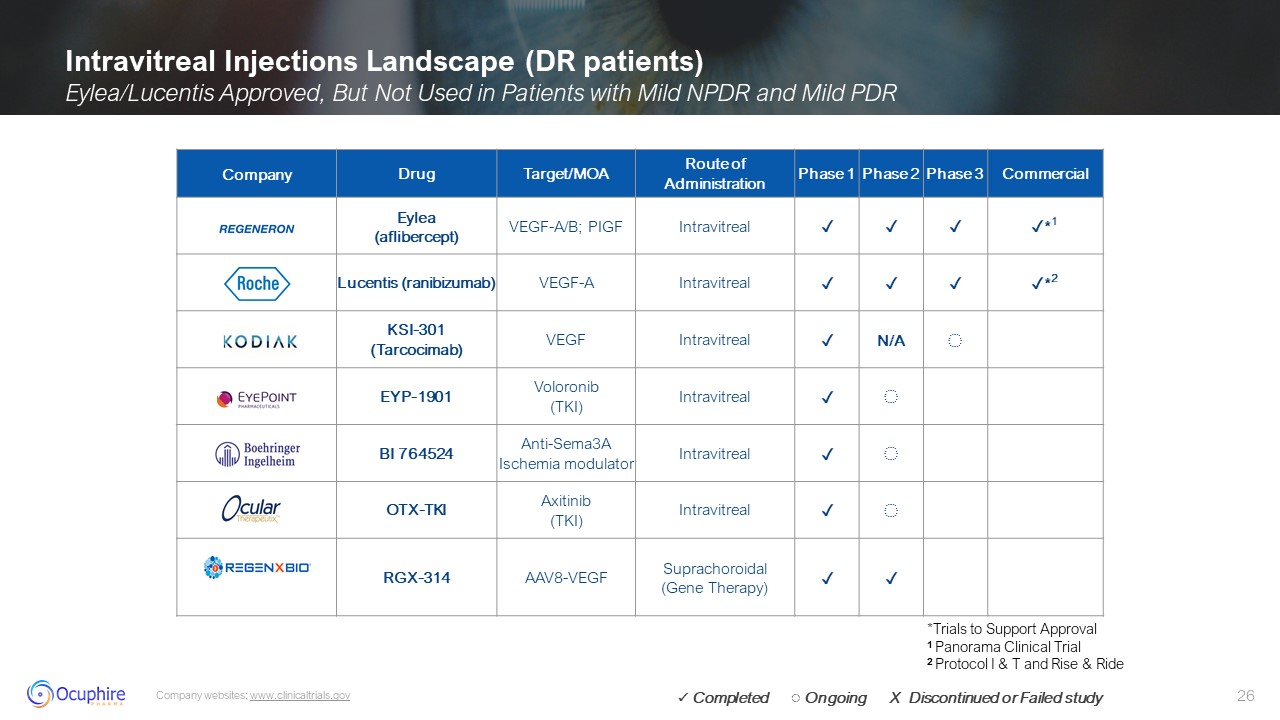
Intravitreal Injections Landscape (DR patients) Eylea/Lucentis Approved, But
Not Used in Patients with Mild NPDR and Mild PDR Company websites; www.clinicaltrials.gov Company Drug Target/MOA Route of Administration Phase 1 Phase 2 Phase 3 Commercial Eylea (aflibercept) VEGF-A/B;
PIGF Intravitreal ✓ ✓ ✓ ✓*1 Lucentis (ranibizumab) VEGF-A Intravitreal ✓ ✓ ✓ ✓*2 KSI-301 (Tarcocimab) VEGF Intravitreal ✓ N/A ◌ EYP-1901 Voloronib (TKI) Intravitreal ✓ ◌ BI 764524 Anti-Sema3A Ischemia
modulator Intravitreal ✓ ◌ OTX-TKI Axitinib (TKI) Intravitreal ✓ ◌ RGX-314 AAV8-VEGF Suprachoroidal (Gene Therapy) ✓ ✓ *Trials to Support Approval 1 Panorama Clinical Trial 2 Protocol I & T and Rise & Ride ✓
Completed ◌ Ongoing X Discontinued or Failed study

Topical Eyedrops in Clinical Development for DR/DME Inflammation MOAs in Phase
2 with Novel Eyedrops Company websites; www.clinicaltrials.gov Company Drug Target/MOA Indication Route of Administration Phase 1 Phase 2 Phase 3 Commercial OCS-01 Steroid DME Eyedrop ✓ ✓ OTT166 Integrin
inhibitor DR Eyedrop ✓ ◌ ✓ Completed ◌ Ongoing X Discontinued or Failed study
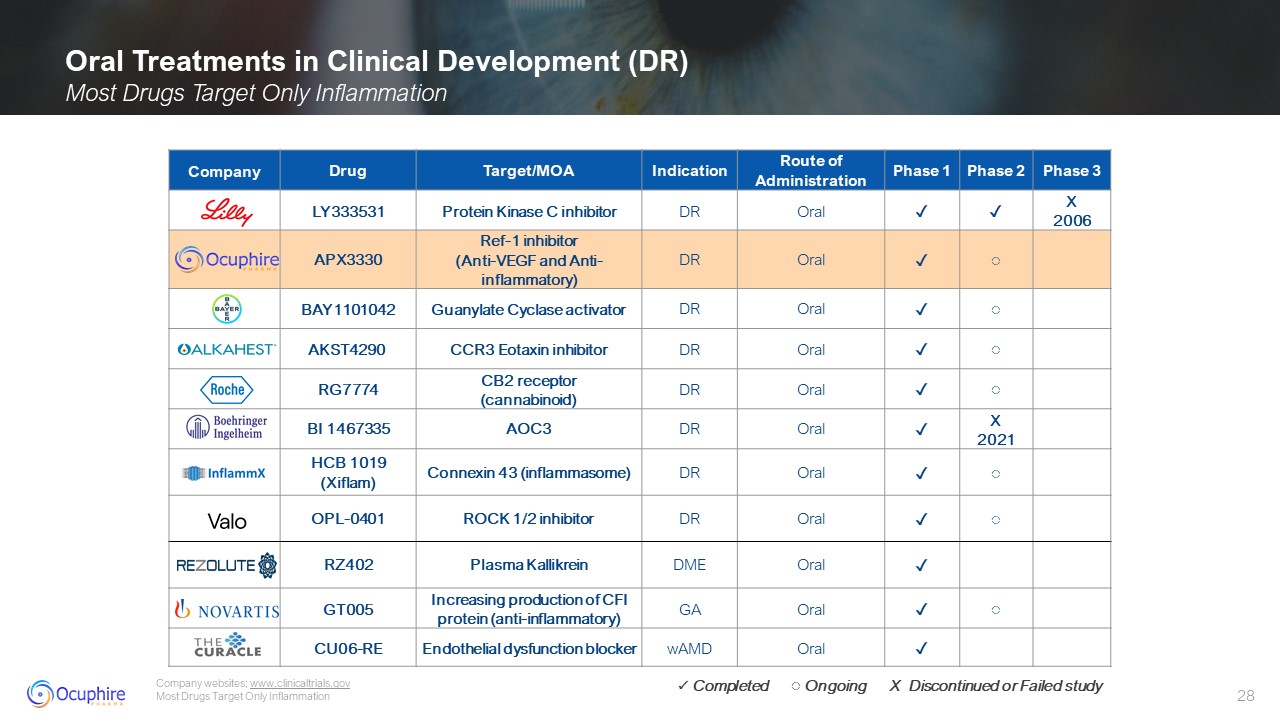
Oral Treatments in Clinical Development (DR) Most Drugs Target Only
Inflammation Company websites; www.clinicaltrials.gov Most Drugs Target Only Inflammation Company Drug Target/MOA Indication Route of Administration Phase 1 Phase 2 Phase 3 LY333531 Protein Kinase C
inhibitor DR Oral ✓ ✓ X 2006 APX3330 Ref-1 inhibitor (Anti-VEGF and Anti-inflammatory) DR Oral ✓ ◌ BAY1101042 Guanylate Cyclase activator DR Oral ✓ ◌ AKST4290 CCR3 Eotaxin inhibitor DR Oral ✓ ◌ RG7774 CB2
receptor (cannabinoid) DR Oral ✓ ◌ BI 1467335 AOC3 DR Oral ✓ X 2021 HCB 1019 (Xiflam) Connexin 43 (inflammasome) DR Oral ✓ ◌ OPL-0401 ROCK 1/2 inhibitor DR Oral ✓ ◌ RZ402 Plasma
Kallikrein DME Oral ✓ GT005 Increasing production of CFI protein (anti-inflammatory) GA Oral ✓ ◌ CU06-RE Endothelial dysfunction blocker wAMD Oral ✓ ✓ Completed ◌ Ongoing X Discontinued or Failed study
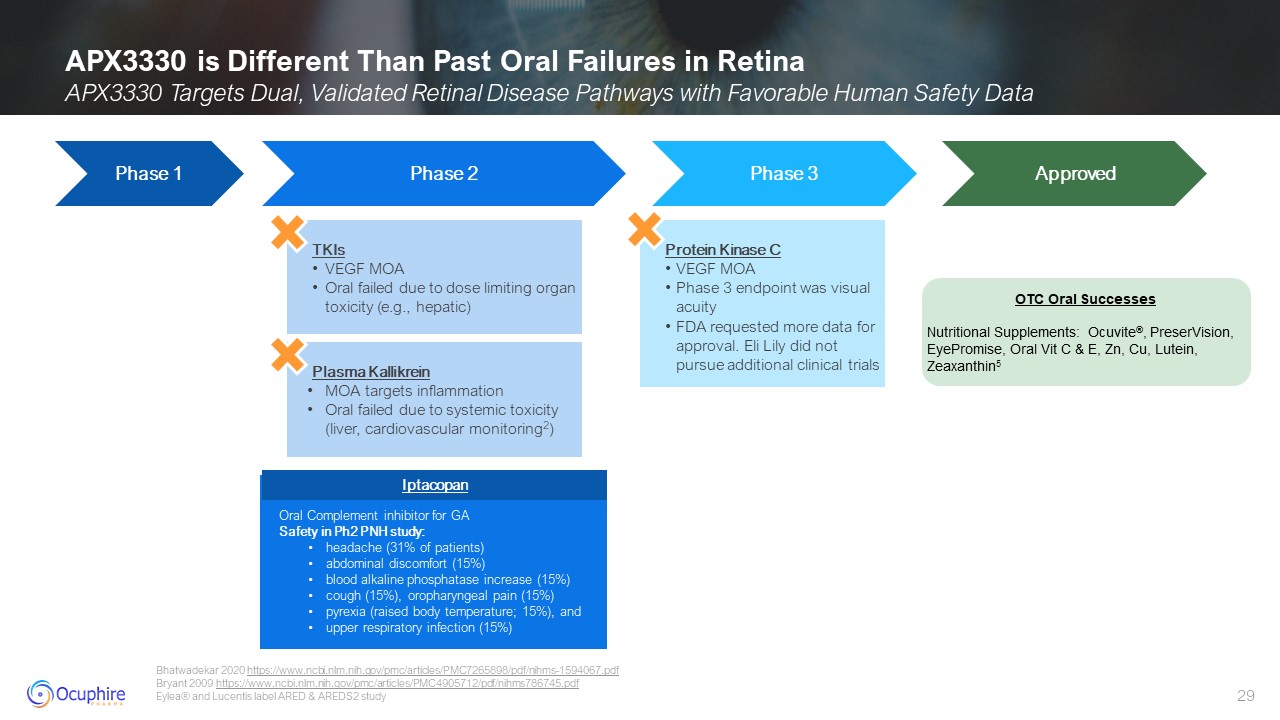
APX3330 is Different Than Past Oral Failures in Retina APX3330 Targets Dual,
Validated Retinal Disease Pathways with Favorable Human Safety Data Bhatwadekar 2020 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7265898/pdf/nihms-1594067.pdf Bryant 2009
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4905712/pdf/nihms786745.pdf Eylea® and Lucentis label ARED & AREDS2 study Phase 3 Commercial Plasma Kallikrein MOA targets inflammation Oral failed due to systemic toxicity (liver,
cardiovascular monitoring2) Protein Kinase C VEGF MOA Phase 3 endpoint was visual acuity FDA requested more data for approval. Eli Lily did not pursue additional clinical trials Phase 1 Phase 2 Phase 3 Approved TKIs VEGF MOA Oral
failed due to dose limiting organ toxicity (e.g., hepatic) OTC Oral Successes Nutritional Supplements: Ocuvite®, PreserVision, EyePromise, Oral Vit C & E, Zn, Cu, Lutein, Zeaxanthin5 Iptacopan Oral Complement inhibitor for GA Safety
in Ph2 PNH study: headache (31% of patients) abdominal discomfort (15%) blood alkaline phosphatase increase (15%) cough (15%), oropharyngeal pain (15%) pyrexia (raised body temperature; 15%), and upper respiratory infection
(15%) Iptacopan

New MOAs/therapies are needed to: Provide non-invasive options for early
disease management Decrease in Diabetic Retinopathy Severity Score (DRSS) Decrease in macular edema Reduce vision threatening complications (VTC) Improve in macular ischemia Improve compliance by longer acting drugs Manage
inflammation Address non-responders APX3330 offers: A novel, dual MOA A novel and non-invasive route, where oral medication allows for early intervention Opportunities for New Therapies in Retina Unmet Needs in Retina Especially in
NPDR

APX3330: Paradigm Shift Oral Treatment Option Presented by: David Lally,
MD Director of the Retina Research Institute at New England Retina Consultants Retina Surgeon at Baystate Medical Center Assistant Professor of Ophthalmology at the University of Massachusetts Medical School-Baystate Published in over 25
peer-reviewed ophthalmic journals and delivered over 25 presentations at national meetings Active member of the American Society of Retina Specialists with the Fellow of the American Society of Retina Specialist (FASRS) award
designation David Lally, MD Jefferson Medical College
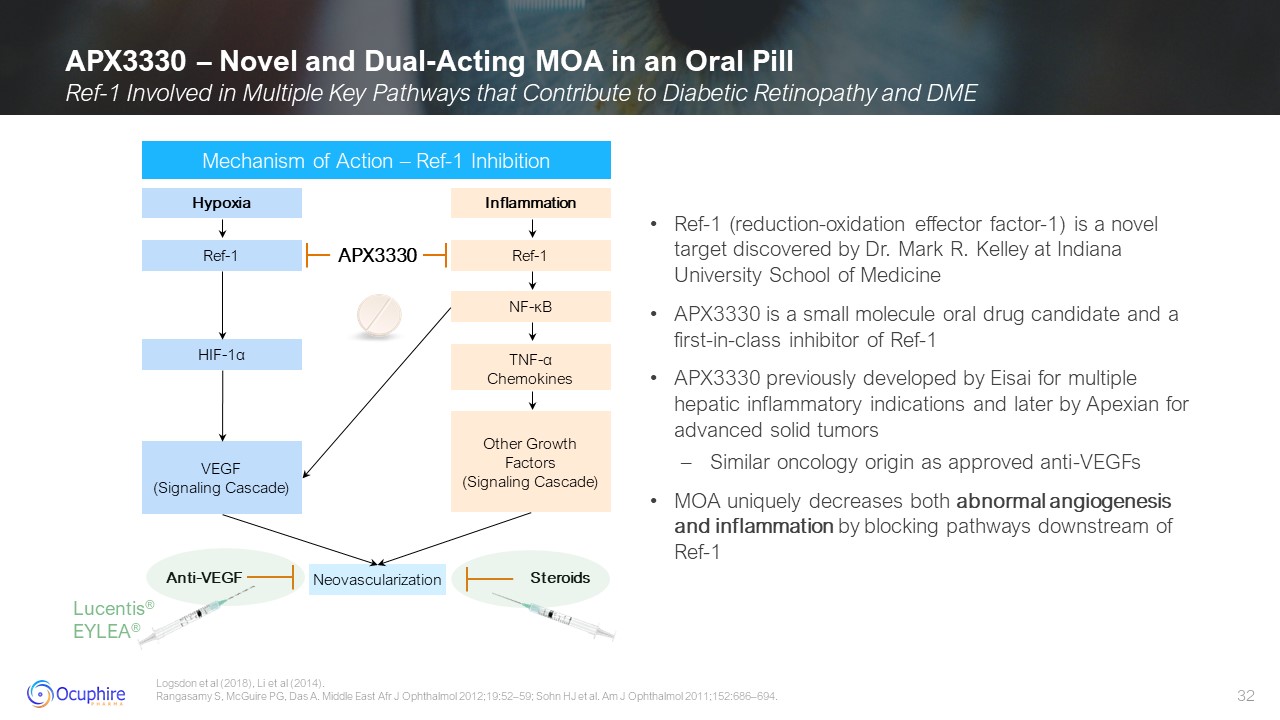
APX3330 – Novel and Dual-Acting MOA in an Oral Pill Ref-1 Involved in Multiple
Key Pathways that Contribute to Diabetic Retinopathy and DME Logsdon et al (2018), Li et al (2014). Rangasamy S, McGuire PG, Das A. Middle East Afr J Ophthalmol 2012;19:52–59; Sohn HJ et al. Am J Ophthalmol 2011;152:686–694. Mechanism of
Action – Ref-1 Inhibition Lucentis® EYLEA® Ref-1 (reduction-oxidation effector factor-1) is a novel target discovered by Dr. Mark R. Kelley at Indiana University School of Medicine APX3330 is a small molecule oral drug candidate and a
first-in-class inhibitor of Ref-1 APX3330 previously developed by Eisai for multiple hepatic inflammatory indications and later by Apexian for advanced solid tumors Similar oncology origin as approved anti-VEGFs MOA uniquely decreases both
abnormal angiogenesis and inflammation by blocking pathways downstream of Ref-1 Hypoxia Ref-1 HIF-1α VEGF (Signaling Cascade) Inflammation Ref-1 NF-κB Other Growth Factors (Signaling
Cascade) TNF-α Chemokines Neovascularization Anti-VEGF Steroids APX3330
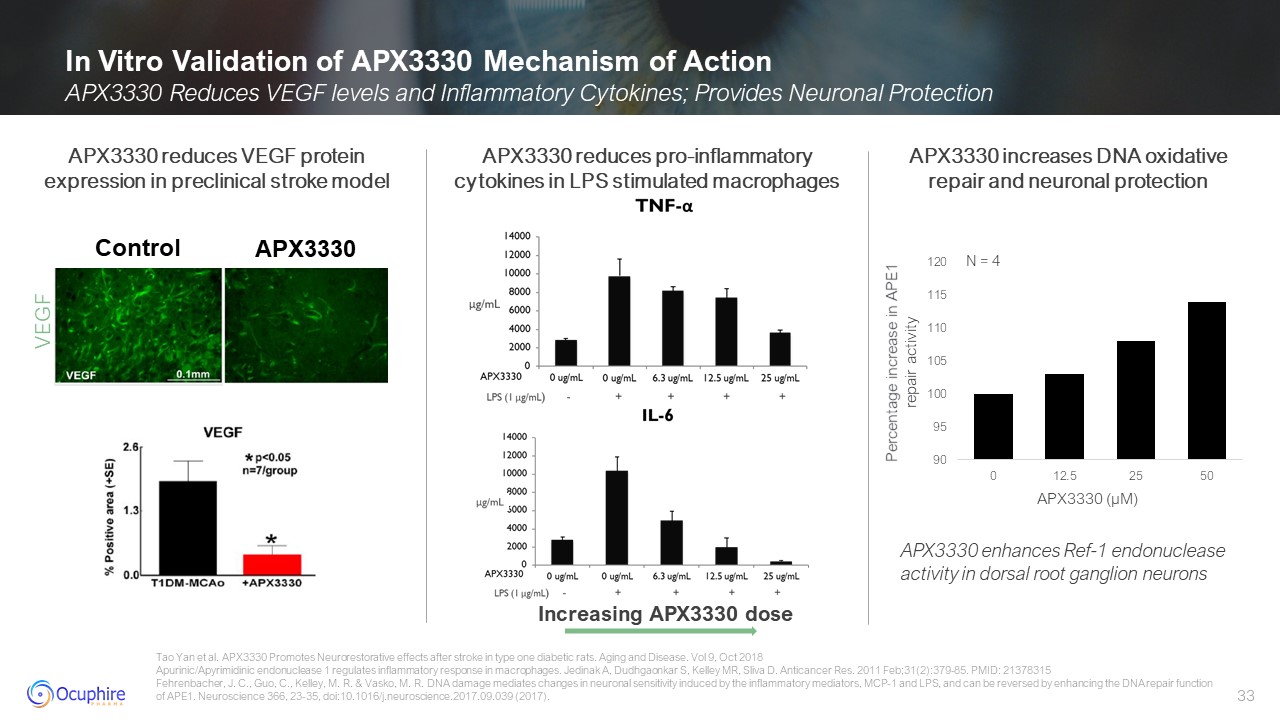
In Vitro Validation of APX3330 Mechanism of Action APX3330 Reduces VEGF levels
and Inflammatory Cytokines; Provides Neuronal Protection Tao Yan et al. APX3330 Promotes Neurorestorative effects after stroke in type one diabetic rats. Aging and Disease. Vol 9, Oct 2018 Apurinic/Apyrimidinic endonuclease 1 regulates
inflammatory response in macrophages. Jedinak A, Dudhgaonkar S, Kelley MR, Sliva D. Anticancer Res. 2011 Feb;31(2):379-85. PMID: 21378315 Fehrenbacher, J. C., Guo, C., Kelley, M. R. & Vasko, M. R. DNA damage mediates changes in neuronal
sensitivity induced by the inflammatory mediators, MCP-1 and LPS, and can be reversed by enhancing the DNA repair function of APE1. Neuroscience 366, 23-35, doi:10.1016/j.neuroscience.2017.09.039 (2017). APX3330 reduces VEGF protein
expression in preclinical stroke model APX3330 reduces pro-inflammatorycytokines in LPS stimulated macrophages Increasing APX3330 dose VEGF Control APX3330 APX3330 increases DNA oxidative repair and neuronal protection APX3330 enhances
Ref-1 endonuclease activity in dorsal root ganglion neurons APX3330 (µM) Percentage increase in APE1 repair activity N = 4
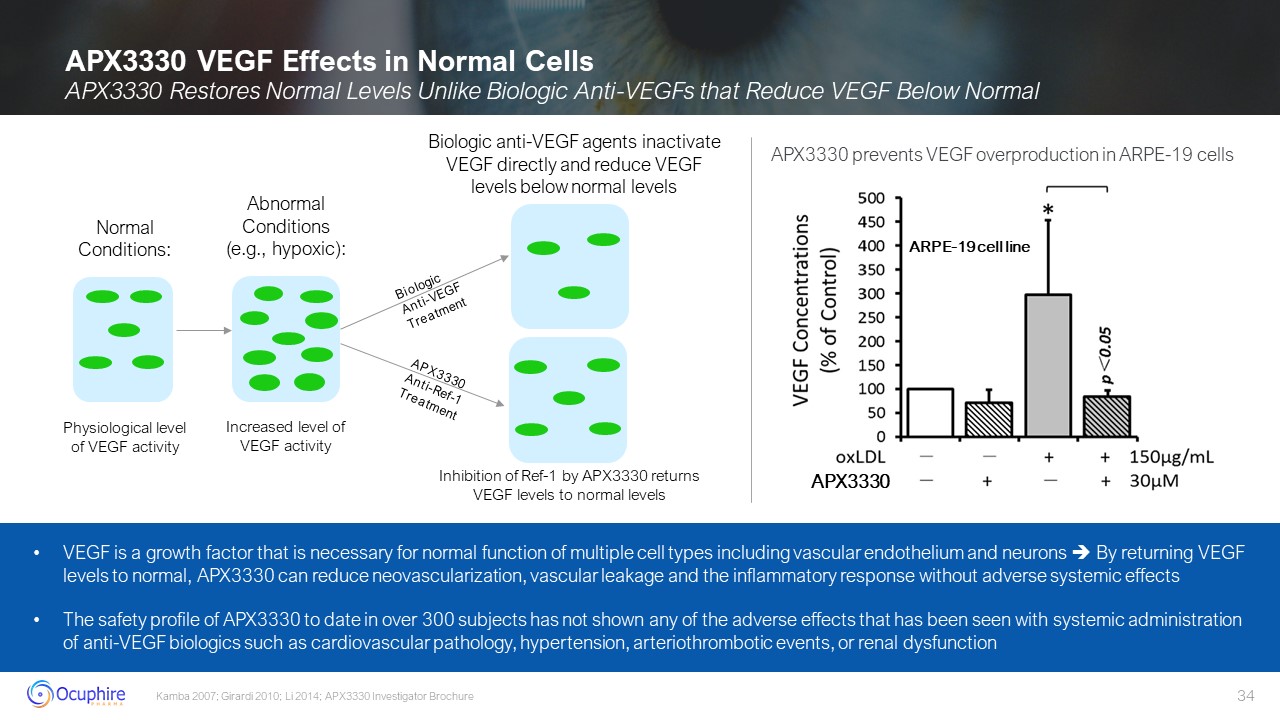
Abnormal Conditions (e.g., hypoxic): Increased level of VEGF activity Normal
Conditions: Physiological level of VEGF activity APX3330 VEGF Effects in Normal Cells APX3330 Restores Normal Levels Unlike Biologic Anti-VEGFs that Reduce VEGF Below Normal Kamba 2007; Girardi 2010; Li 2014; APX3330 Investigator
Brochure Biologic Anti-VEGF Treatment APX3330 Anti-Ref-1 Treatment Biologic anti-VEGF agents inactivate VEGF directly and reduce VEGF levels below normal levels Inhibition of Ref-1 by APX3330 returns VEGF levels to normal levels VEGF is
a growth factor that is necessary for normal function of multiple cell types including vascular endothelium and neurons By returning VEGF levels to normal, APX3330 can reduce neovascularization, vascular leakage and the inflammatory
response without adverse systemic effects The safety profile of APX3330 to date in over 300 subjects has not shown any of the adverse effects that has been seen with systemic administration of anti-VEGF biologics such as cardiovascular
pathology, hypertension, arteriothrombotic events, or renal dysfunction APX3330 ARPE-19 cell line APX3330 prevents VEGF overproduction in ARPE-19 cells
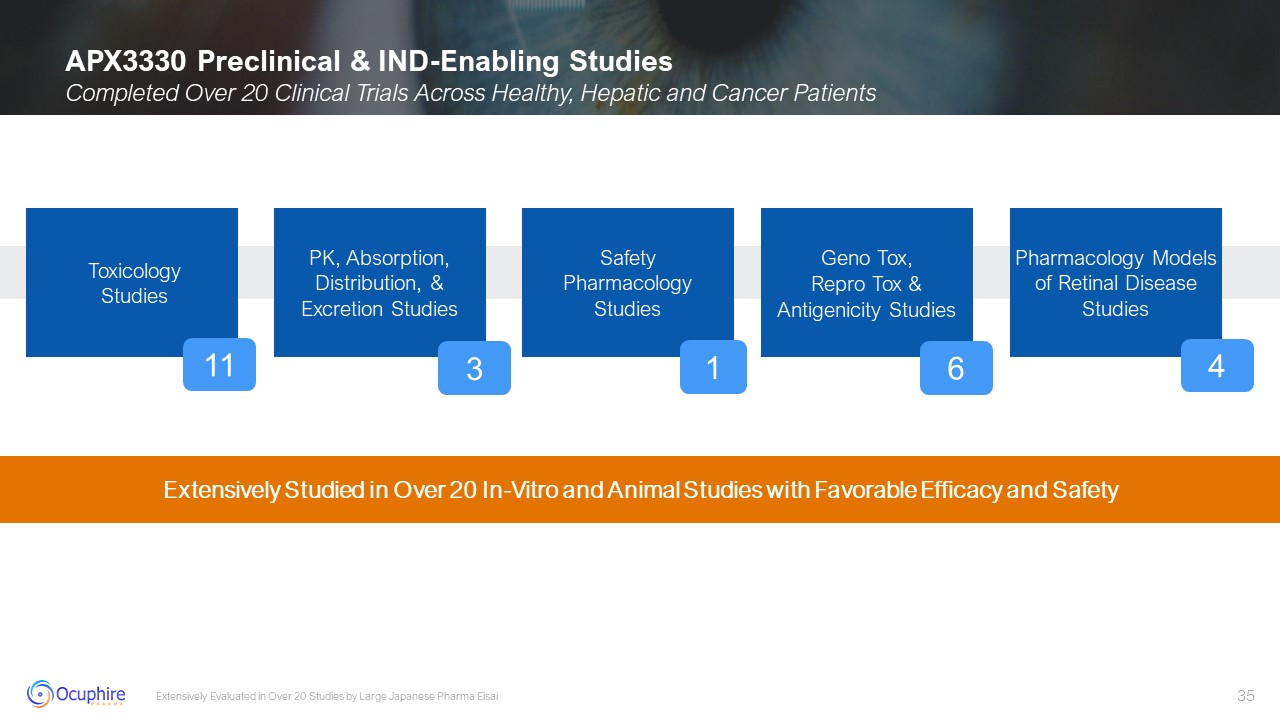
APX3330 Preclinical & IND-Enabling Studies Completed Over 20 Clinical
Trials Across Healthy, Hepatic and Cancer Patients Extensively Evaluated in Over 20 Studies by Large Japanese Pharma Eisai Toxicology Studies PK, Absorption, Distribution, & Excretion Studies Safety Pharmacology Studies Geno Tox,
Repro Tox & Antigenicity Studies Pharmacology Models of Retinal Disease Studies 11 1 6 4 3 Extensively Studied in Over 20 In-Vitro and Animal Studies with Favorable Efficacy and Safety
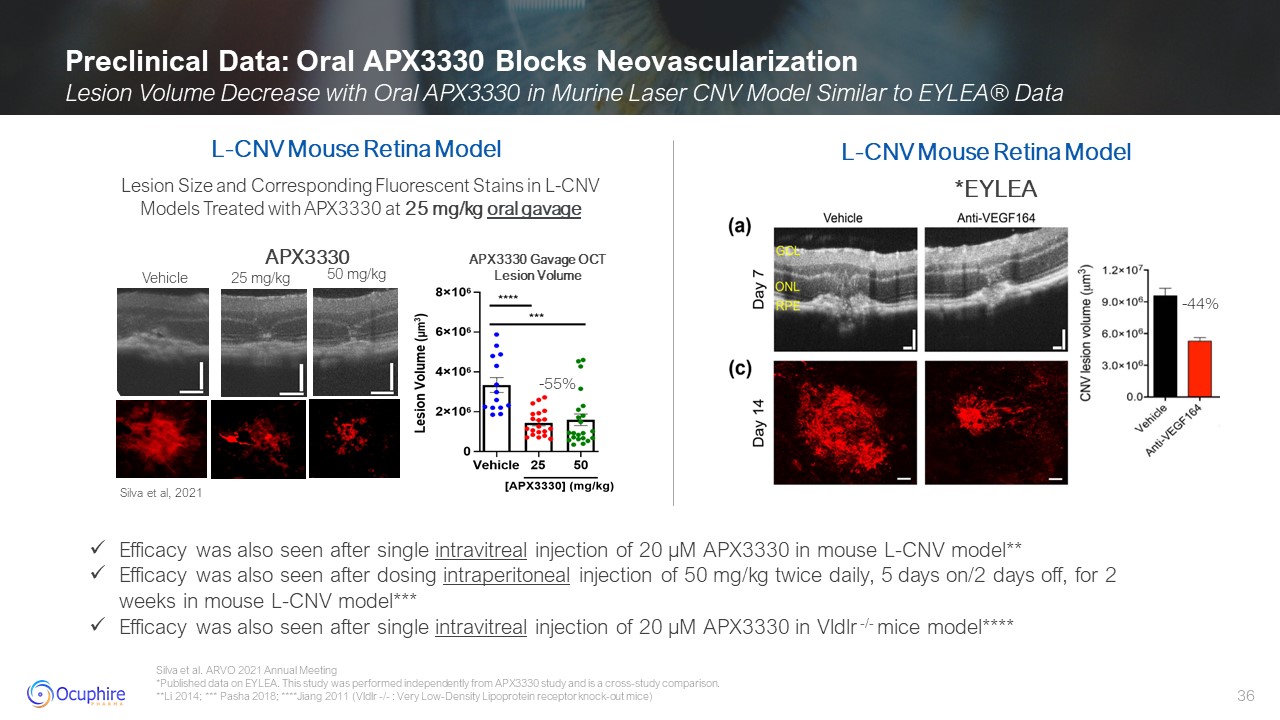
Silva et al. ARVO 2021 Annual Meeting *Published data on EYLEA. This study was
performed independently from APX3330 study and is a cross-study comparison. **Li 2014; *** Pasha 2018; ****Jiang 2011 (Vldlr -/- : Very Low-Density Lipoprotein receptor knock-out mice) Preclinical Data: Oral APX3330 Blocks
Neovascularization Lesion Volume Decrease with Oral APX3330 in Murine Laser CNV Model Similar to EYLEA® Data *EYLEA Lesion Size and Corresponding Fluorescent Stains in L-CNV Models Treated with APX3330 at 25 mg/kg oral gavage -55% L-CNV
Mouse Retina Model Silva et al, 2021 Vehicle 25 mg/kg 50 mg/kg APX3330 Gavage OCT Lesion Volume L-CNV Mouse Retina Model APX3330 Efficacy was also seen after single intravitreal injection of 20 µM APX3330 in mouse L-CNV
model** Efficacy was also seen after dosing intraperitoneal injection of 50 mg/kg twice daily, 5 days on/2 days off, for 2 weeks in mouse L-CNV model*** Efficacy was also seen after single intravitreal injection of 20 µM APX3330 in Vldlr
-/- mice model**** -44%

Summary of APX3330 Prior Clinical Trials Completed 11 Clinical Trials Across
Healthy, Hepatic and Cancer Patients Phase 1 Study ID Patient Population Treatment Groups APX_CLN_0001 Healthy Subjects APX3330, Placebo APX_CLN_0002 Healthy Subjects APX3330, Placebo APX_CLN_0003 Healthy
Subjects APX3330 APX_CLN_0004 Healthy Subjects APX3330 APX_CLN_0008 Healthy Subjects APX3330, Placebo Phase 2 Study ID Patient Population Treatment Groups APX_CLN_0005 Chronic Hep B APX3330 APX_CLN_0006 Chronic
Hep C APX3330 APX_CLN_0007 Chronic Hep C APX3330, Placebo APX_CLN_0009 Acute severe hepatitis APX3330 APX_CLN_0010 Alcoholic hepatitis APX3330 APX_CLN_0011 Cancer (solid tumors) APX3330 Phase 1 Studies Phase 2
Studies Extensively Studied in 11 Clinical Trials across Phase 1 and Phase 2 by Eisai and Apexian 5 6
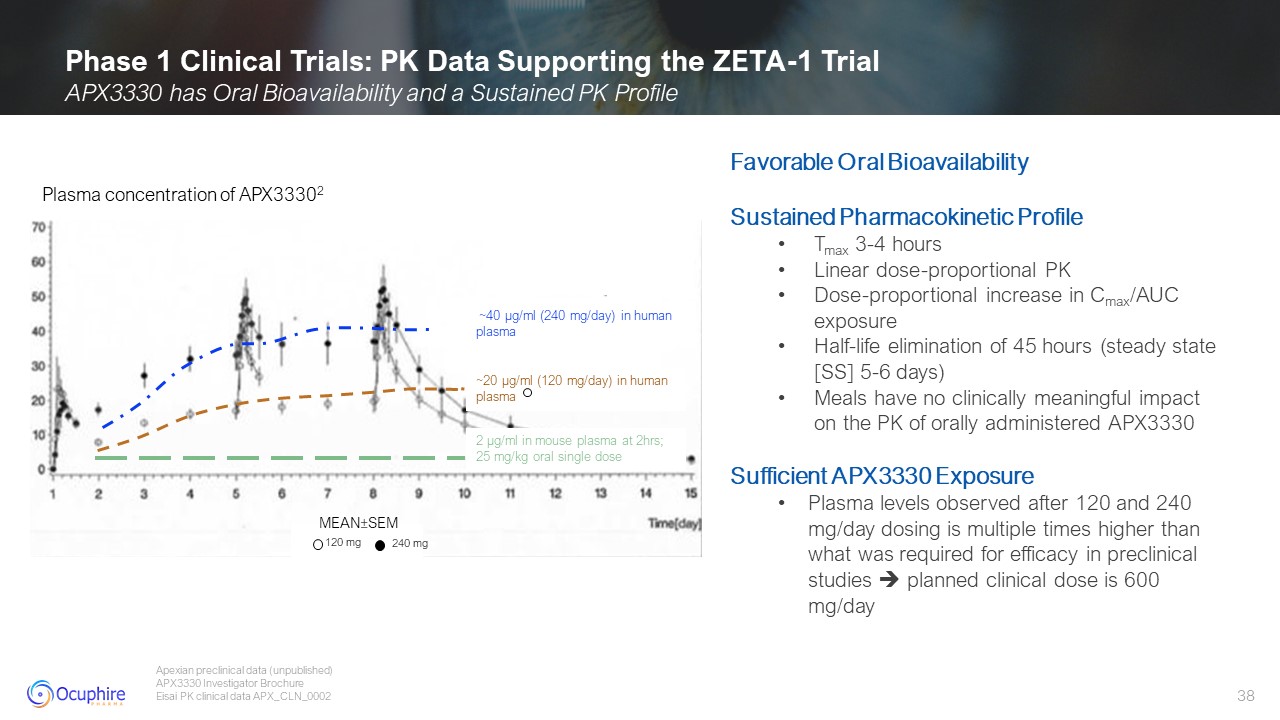
Phase 1 Clinical Trials: PK Data Supporting the ZETA-1 Trial APX3330 has Oral
Bioavailability and a Sustained PK Profile Apexian preclinical data (unpublished) APX3330 Investigator Brochure Eisai PK clinical data APX_CLN_0002 Favorable Oral Bioavailability Sustained Pharmacokinetic Profile Tmax 3-4
hours Linear dose-proportional PK Dose-proportional increase in Cmax/AUC exposure Half-life elimination of 45 hours (steady state [SS] 5-6 days) Meals have no clinically meaningful impact on the PK of orally administered
APX3330 Sufficient APX3330 Exposure Plasma levels observed after 120 and 240 mg/day dosing is multiple times higher than what was required for efficacy in preclinical studies planned clinical dose is 600 mg/day 2 µg/ml in mouse plasma at
2hrs; 25 mg/kg oral single dose ~20 µg/ml (120 mg/day) in human plasma ~40 µg/ml (240 mg/day) in human plasma Plasma concentration of APX33302 z MEAN±SEM 120 mg 240 mg
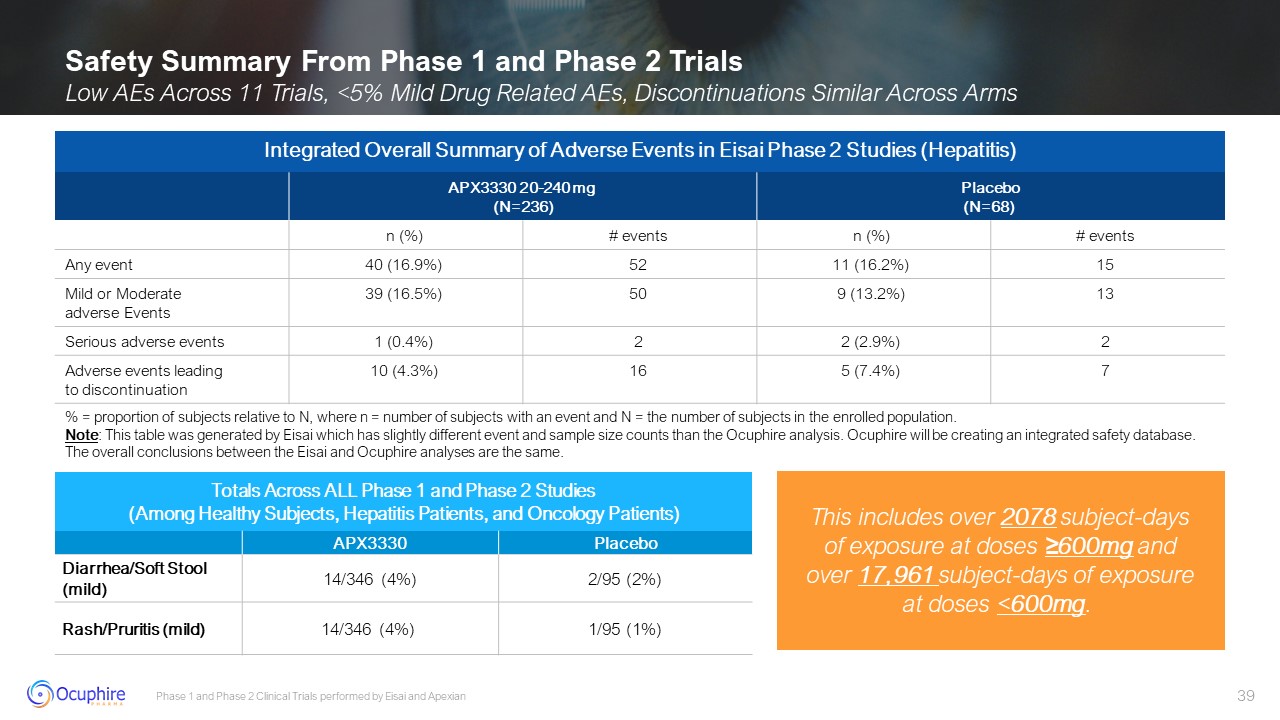
Phase 1 and Phase 2 Clinical Trials performed by Eisai and Apexian Safety
Summary From Phase 1 and Phase 2 Trials Low AEs Across 11 Trials, <5% Mild Drug Related AEs, Discontinuations Similar Across Arms Totals Across ALL Phase 1 and Phase 2 Studies (Among Healthy Subjects, Hepatitis Patients, and Oncology
Patients) APX3330 Placebo Diarrhea/Soft Stool (mild) 14/346 (4%) 2/95 (2%) Rash/Pruritis (mild) 14/346 (4%) 1/95 (1%) Integrated Overall Summary of Adverse Events in Eisai Phase 2 Studies (Hepatitis) APX3330 20-240
mg (N=236) Placebo (N=68) n (%) # events n (%) # events Any event 40 (16.9%) 52 11 (16.2%) 15 Mild or Moderate adverse Events 39 (16.5%) 50 9 (13.2%) 13 Serious adverse events 1 (0.4%) 2 2
(2.9%) 2 Adverse events leadingto discontinuation 10 (4.3%) 16 5 (7.4%) 7 % = proportion of subjects relative to N, where n = number of subjects with an event and N = the number of subjects in the enrolled population. Note:
This table was generated by Eisai which has slightly different event and sample size counts than the Ocuphire analysis. Ocuphire will be creating an integrated safety database. The overall conclusions between the Eisai and Ocuphire analyses
are the same. This includes over 2078 subject-days of exposure at doses ≥600mg and over 17,961 subject-days of exposure at doses <600mg.

ZETA-1 Phase 2b Trial in Diabetic Retinopathy Presented by: David Lally,
MD David Lally, MD Jefferson Medical College
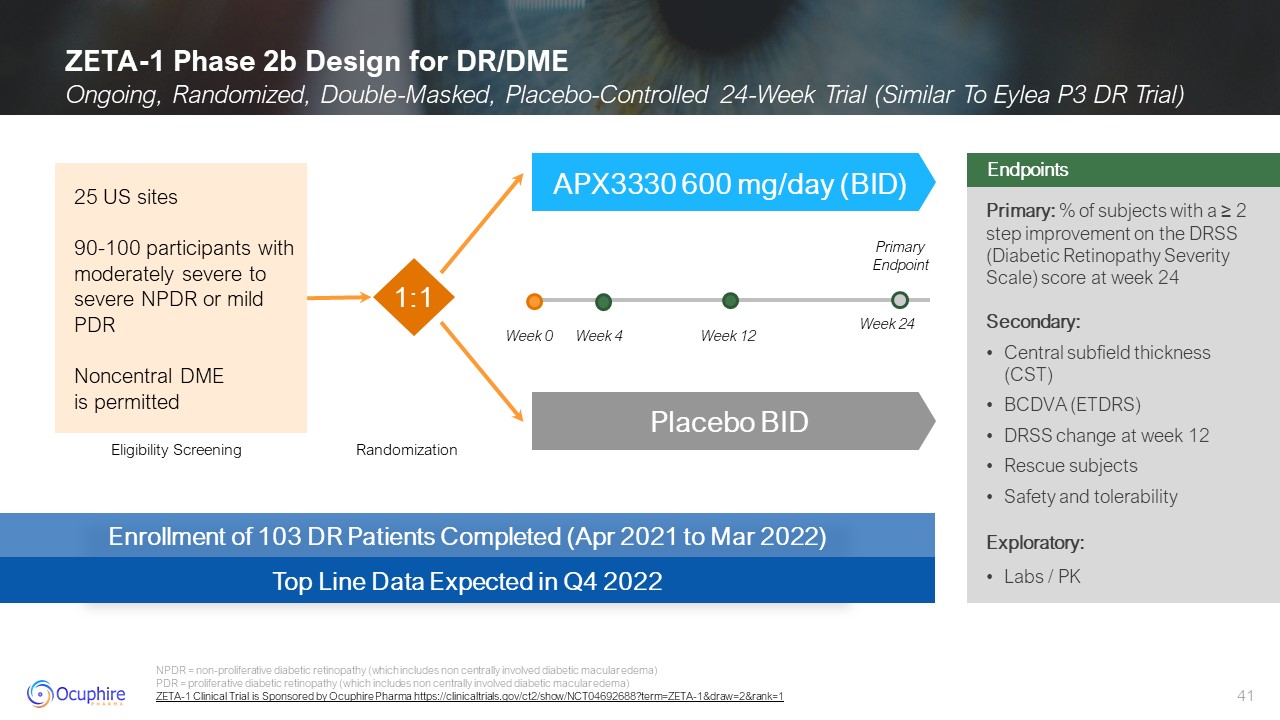
ZETA-1 Phase 2b Design for DR/DME Ongoing, Randomized, Double-Masked,
Placebo-Controlled 24-Week Trial (Similar To Eylea P3 DR Trial) NPDR = non-proliferative diabetic retinopathy (which includes non centrally involved diabetic macular edema) PDR = proliferative diabetic retinopathy (which includes non
centrally involved diabetic macular edema) ZETA-1 Clinical Trial is Sponsored by Ocuphire Pharma https://clinicaltrials.gov/ct2/show/NCT04692688?term=ZETA-1&draw=2&rank=1 Primary: % of subjects with a ≥ 2 step improvement on the
DRSS (Diabetic Retinopathy Severity Scale) score at week 24 Secondary: Central subfield thickness (CST) BCDVA (ETDRS) DRSS change at week 12 Rescue subjects Safety and tolerability Exploratory: Labs / PK Endpoints Enrollment of 103
DR Patients Completed (Apr 2021 to Mar 2022) Top Line Data Expected in Q4 2022 Eligibility Screening Randomization APX3330 600 mg/day (BID) Placebo BID 25 US sites 90-100 participants with moderately severe to severe NPDR or mild
PDR Noncentral DME is permitted 1:1 Week 0 Week 12 Week 24 Week 4 Primary Endpoint
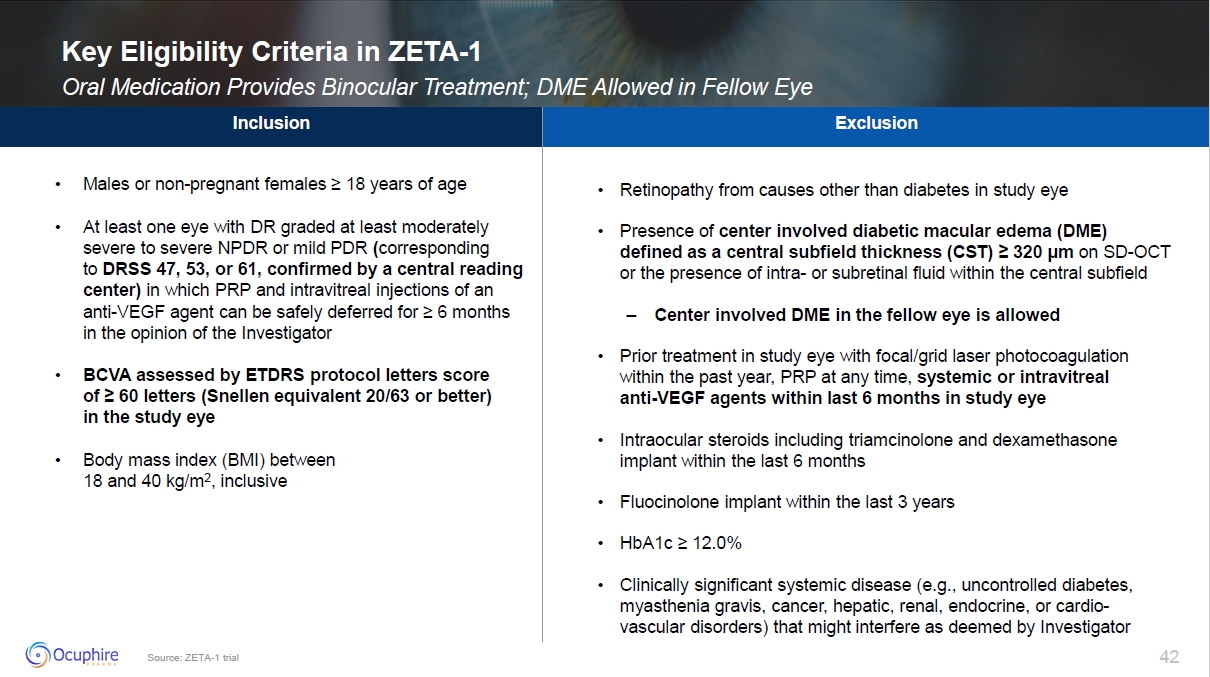
Source: ZETA-1 trial Key Eligibility Criteria in ZETA-1 Oral Medication
Provides Binocular Treatment; DME Allowed in Fellow Eye
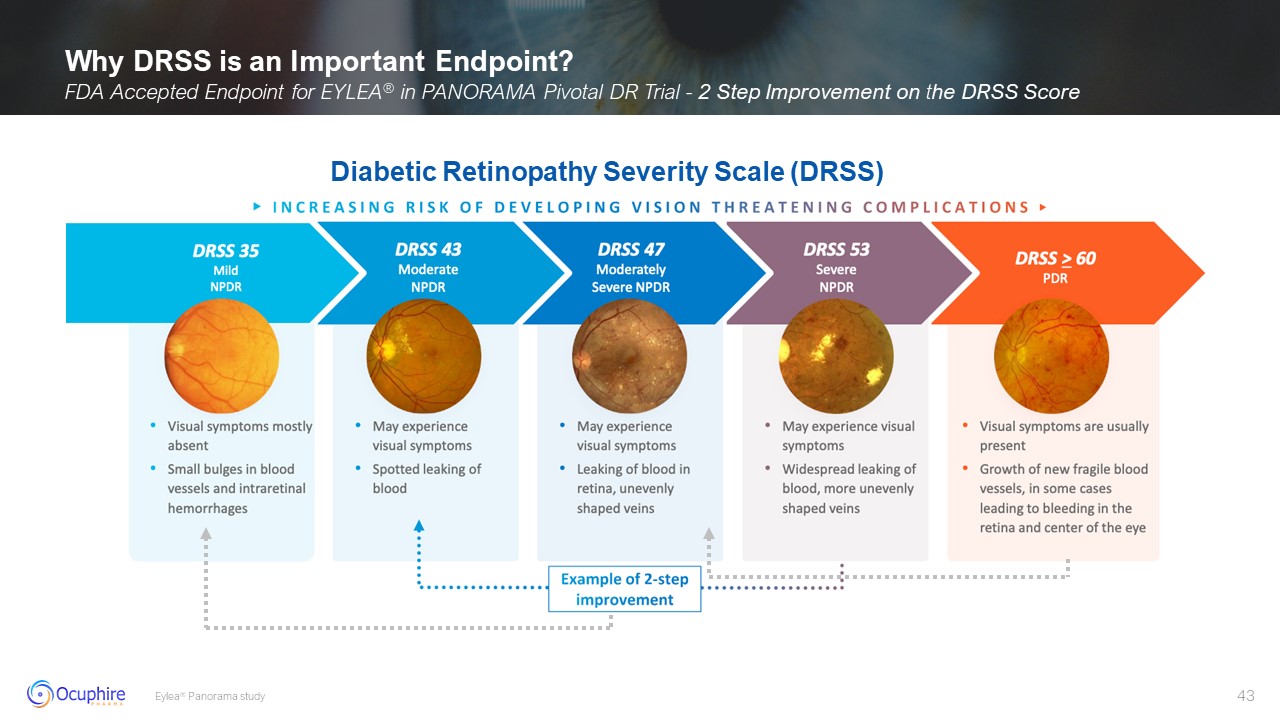
Why DRSS is an Important Endpoint? FDA Accepted Endpoint for EYLEA® in PANORAMA
Pivotal DR Trial - 2 Step Improvement on the DRSS Score Eylea® Panorama study Diabetic Retinopathy Severity Scale (DRSS)

ZETA-1 Trial: Demographics and Masked Safety Data Presented by: Caroline
Baumal, MD Caroline Baumal, MD University of Toronto
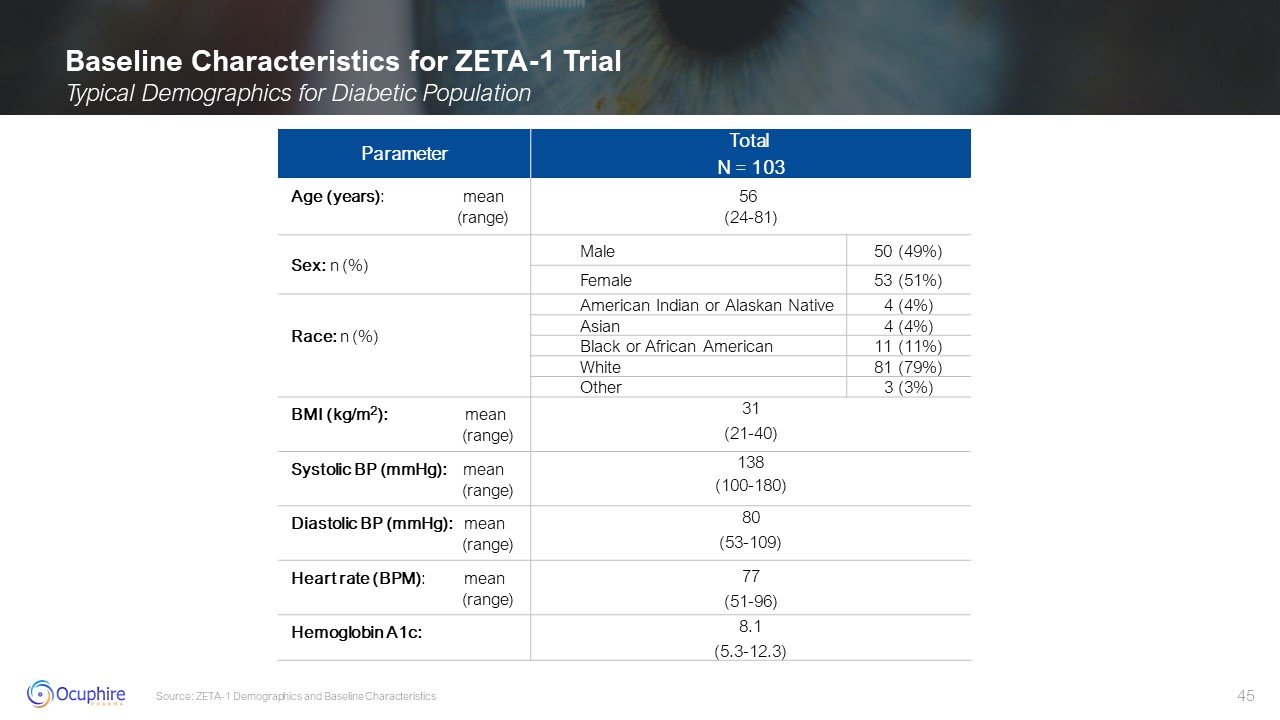
Baseline Characteristics for ZETA-1 Trial Typical Demographics for Diabetic
Population Source: ZETA-1 Demographics and Baseline Characteristics Parameter Total N = 103 Age (years): mean (range) 56 (24-81) Sex: n (%) Male 50 (49%) Female 53
(51%) Race: n (%) American Indian or Alaskan Native 4 (4%) Asian 4 (4%) Black or African American 11 (11%) White 81 (79%) Other 3 (3%) BMI (kg/m2): mean
(range) 31 (21-40) Systolic BP (mmHg): mean (range) 138 (100-180) Diastolic BP (mmHg): mean (range) 80 (53-109) Heart rate (BPM):
mean (range) 77 (51-96) Hemoglobin A1c: 8.1 (5.3-12.3)
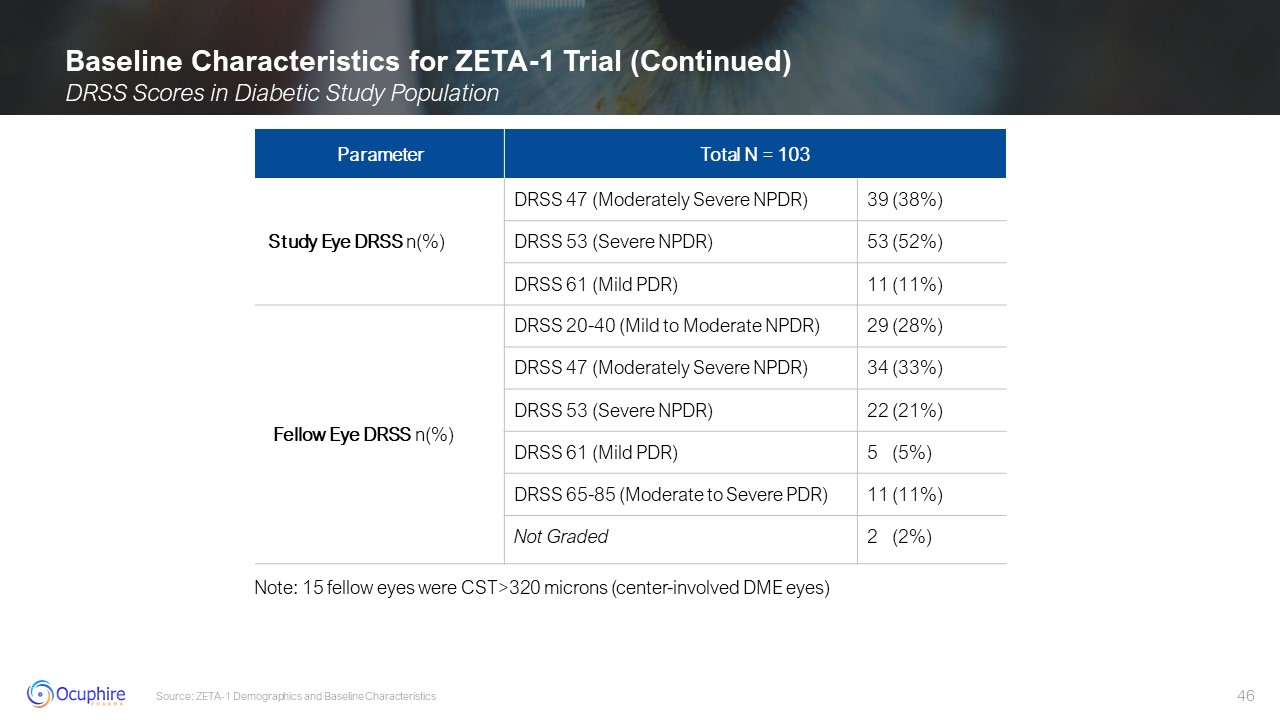
Baseline Characteristics for ZETA-1 Trial (Continued) DRSS Scores in Diabetic
Study Population Source: ZETA-1 Demographics and Baseline Characteristics Parameter Total N = 103 Total N = 103 Study Eye DRSS n(%) DRSS 47 (Moderately Severe NPDR) 39 (38%) DRSS 53 (Severe NPDR) 53 (52%) DRSS 61 (Mild PDR) 11
(11%) Fellow Eye DRSS n(%) DRSS 20-40 (Mild to Moderate NPDR) 29 (28%) DRSS 47 (Moderately Severe NPDR) 34 (33%) DRSS 53 (Severe NPDR) 22 (21%) DRSS 61 (Mild PDR) 5 (5%) DRSS 65-85 (Moderate to Severe PDR) 11 (11%) Not Graded 2
(2%) Note: 15 fellow eyes were CST>320 microns (center-involved DME eyes)
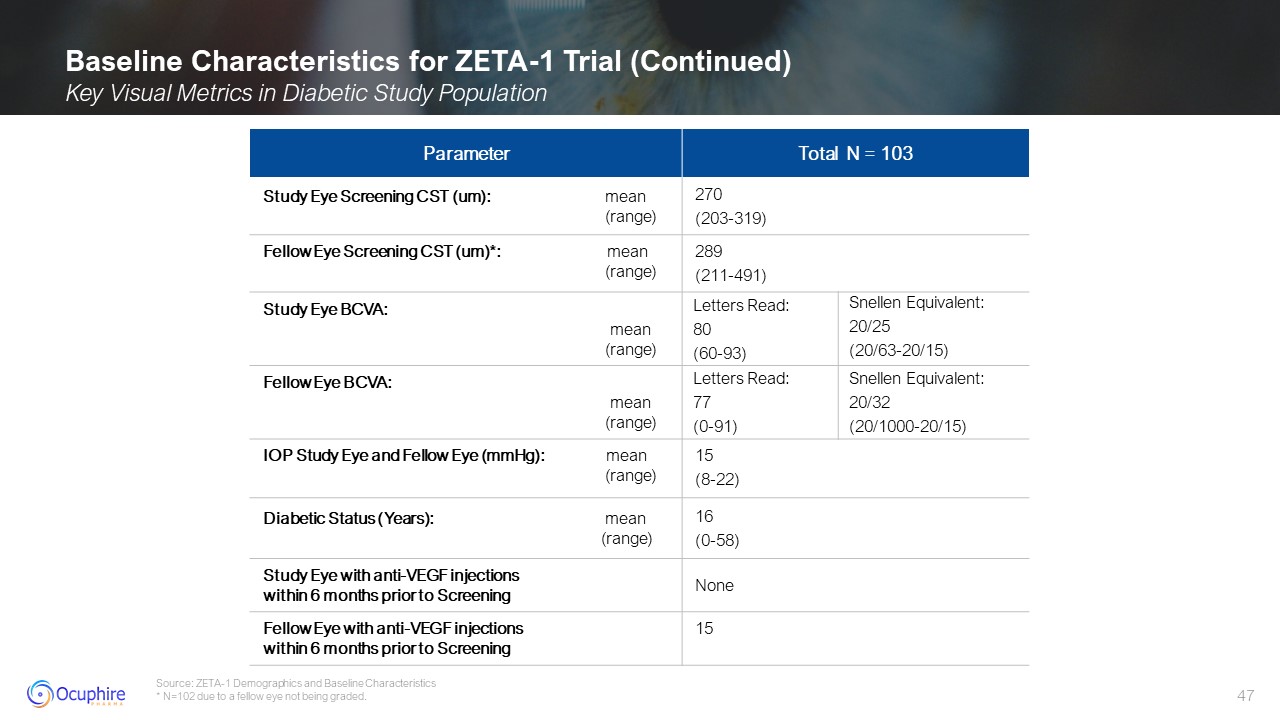
Baseline Characteristics for ZETA-1 Trial (Continued) Key Visual Metrics in
Diabetic Study Population Source: ZETA-1 Demographics and Baseline Characteristics * N=102 due to a fellow eye not being graded. Parameter Total N = 103 Study Eye Screening CST (um): mean (range) 270 (203-319) Fellow Eye
Screening CST (um)*: mean (range) 289 (211-491) Study Eye BCVA: mean (range) Letters Read: 80 (60-93) Snellen Equivalent: 20/25 (20/63-20/15) Fellow Eye BCVA: mean (range) Letters Read: 77 (0-91) Snellen
Equivalent: 20/32 (20/1000-20/15) IOP Study Eye and Fellow Eye (mmHg): mean (range) 15 (8-22) Diabetic Status (Years): mean (range) 16 (0-58) Study Eye with anti-VEGF injectionswithin 6 months prior to Screening None Fellow
Eye with anti-VEGF injectionswithin 6 months prior to Screening 15
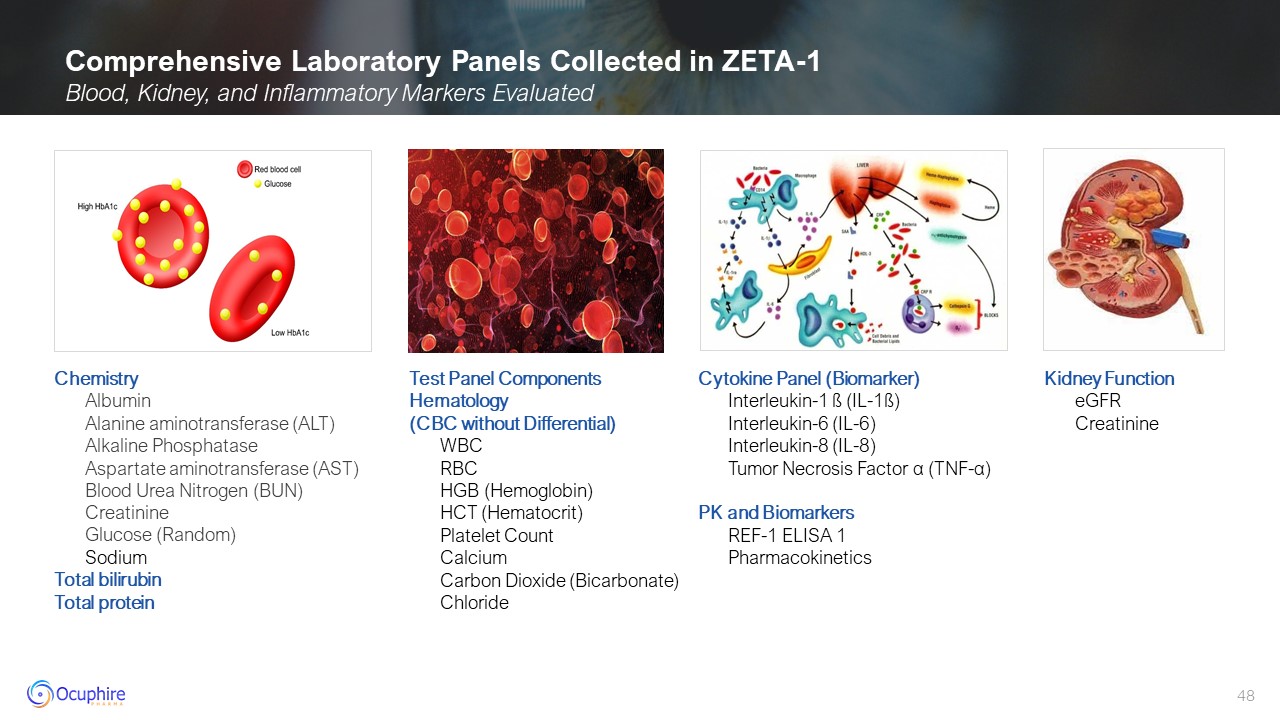
Comprehensive Laboratory Panels Collected in ZETA-1 Blood, Kidney, and
Inflammatory Markers Evaluated Chemistry Albumin Alanine aminotransferase (ALT) Alkaline Phosphatase Aspartate aminotransferase (AST) Blood Urea Nitrogen (BUN) Creatinine Glucose (Random) Sodium Total bilirubin Total
protein Test Panel ComponentsHematology(CBC without Differential) WBC RBC HGB (Hemoglobin) HCT (Hematocrit) Platelet Count Calcium Carbon Dioxide (Bicarbonate) Chloride Cytokine Panel (Biomarker) Interleukin-1 ß
(IL-1ß) Interleukin-6 (IL-6) Interleukin-8 (IL-8) Tumor Necrosis Factor α (TNF-α) PK and Biomarkers REF-1 ELISA 1 Pharmacokinetics Kidney Function eGFR Creatinine
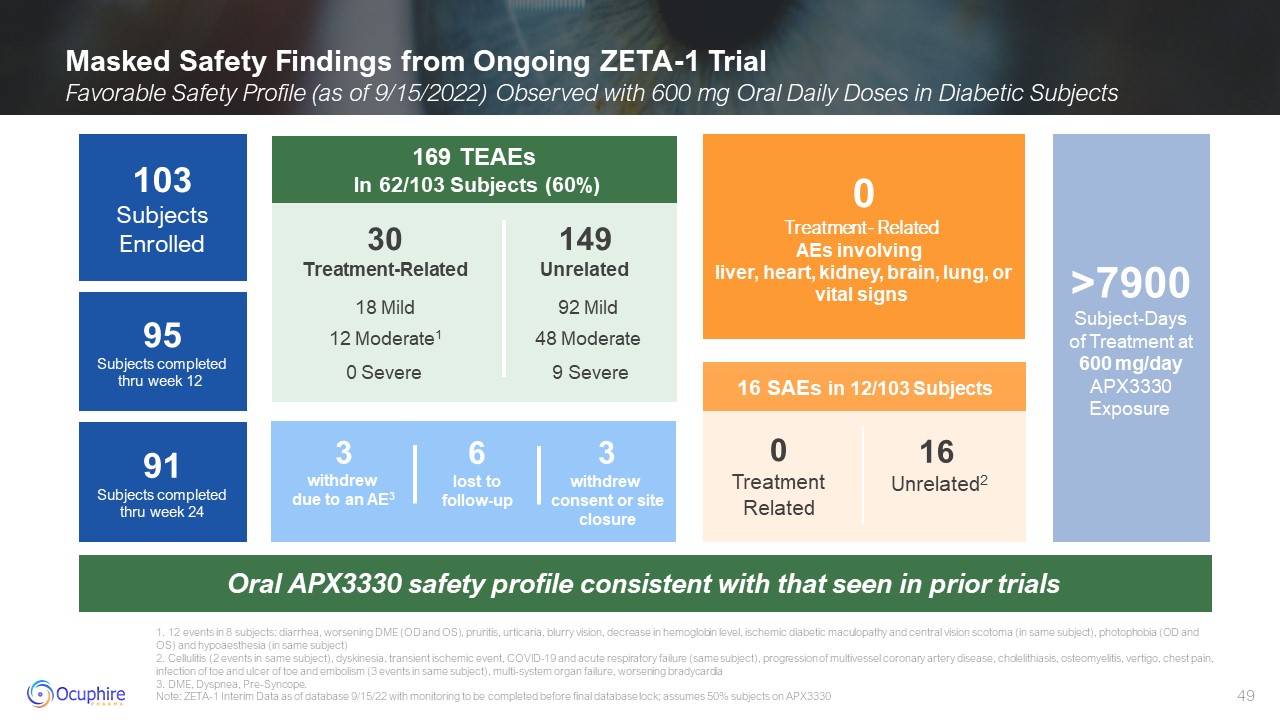
Masked Safety Findings from Ongoing ZETA-1 Trial Favorable Safety Profile (as
of 9/15/2022) Observed with 600 mg Oral Daily Doses in Diabetic Subjects 1. 12 events in 8 subjects: diarrhea, worsening DME (OD and OS), pruritis, urticaria, blurry vision, decrease in hemoglobin level, ischemic diabetic maculopathy and
central vision scotoma (in same subject), photophobia (OD and OS) and hypoaesthesia (in same subject) 2. Cellulitis (2 events in same subject), dyskinesia, transient ischemic event, COVID-19 and acute respiratory failure (same subject),
progression of multivessel coronary artery disease, cholelithiasis, osteomyelitis, vertigo, chest pain, infection of toe and ulcer of toe and embolism (3 events in same subject), multi-system organ failure, worsening bradycardia 3. DME,
Dyspnea, Pre-Syncope. Note: ZETA-1 Interim Data as of database 9/15/22 with monitoring to be completed before final database lock; assumes 50% subjects on APX3330 0 Treatment- Related AEs involving liver, heart, kidney, brain, lung,
or vital signs Oral APX3330 safety profile consistent with that seen in prior trials >7900Subject-Daysof Treatment at600 mg/day APX3330 Exposure 16 SAEs in 12/103 Subjects 0 Treatment Related 16 Unrelated2 91Subjects completed
thru week 24 103 Subjects Enrolled 95Subjects completed thru week 12 169 TEAEs In 62/103 Subjects (60%) 149 Unrelated 30 Treatment-Related 12 Moderate1 0 Severe 92 Mild 48 Moderate 9 Severe 18 Mild 3 withdrew due to
an AE3 3 withdrew consent or site closure 6 lost to follow-up
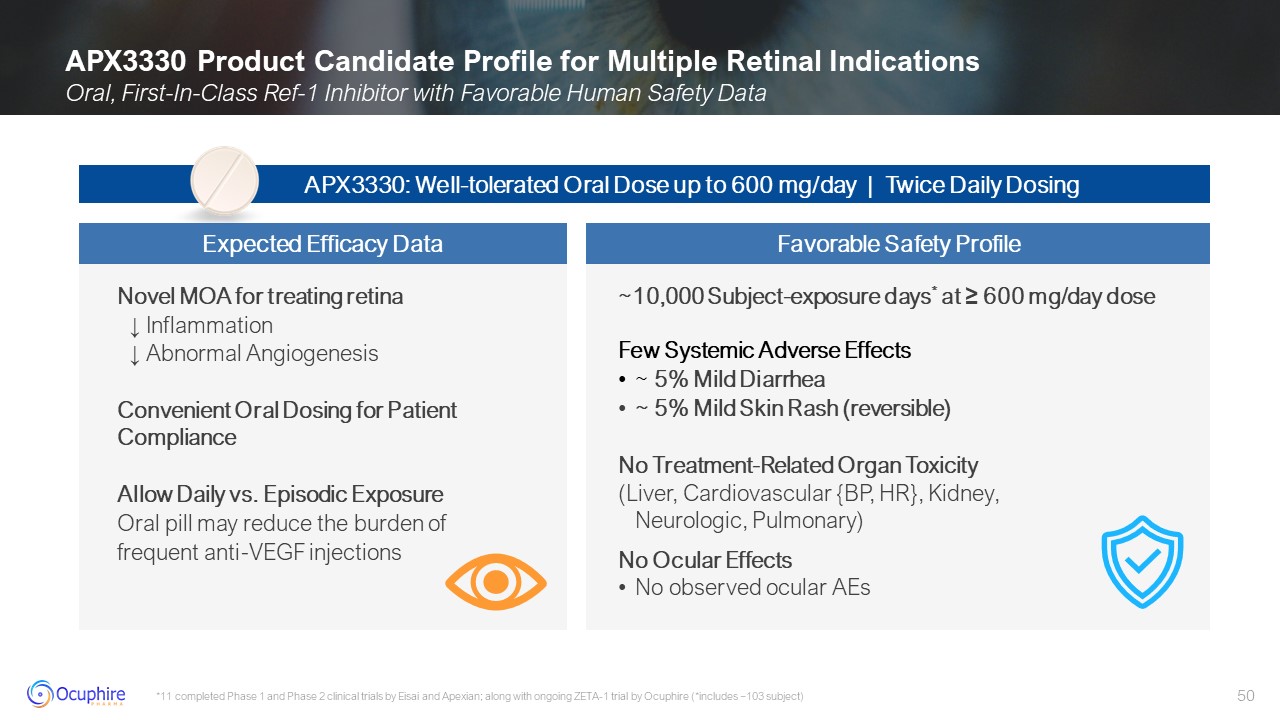
APX3330 Product Candidate Profile for Multiple Retinal Indications Oral,
First-In-Class Ref-1 Inhibitor with Favorable Human Safety Data *11 completed Phase 1 and Phase 2 clinical trials by Eisai and Apexian; along with ongoing ZETA-1 trial by Ocuphire (*includes ~103 subject) Novel MOA for treating retina ↓
Inflammation ↓ Abnormal Angiogenesis Convenient Oral Dosing for Patient Compliance Allow Daily vs. Episodic Exposure Oral pill may reduce the burden of frequent anti-VEGF injections ~10,000 Subject-exposure days* at ≥ 600 mg/day
dose Few Systemic Adverse Effects ~ 5% Mild Diarrhea ~ 5% Mild Skin Rash (reversible) No Treatment-Related Organ Toxicity (Liver, Cardiovascular {BP, HR}, Kidney,Neurologic, Pulmonary) No Ocular Effects No observed ocular
AEs APX3330: Well-tolerated Oral Dose up to 600 mg/day | Twice Daily Dosing Expected Efficacy Data Favorable Safety Profile

ZETA-1 Trial Design and Data Expectations
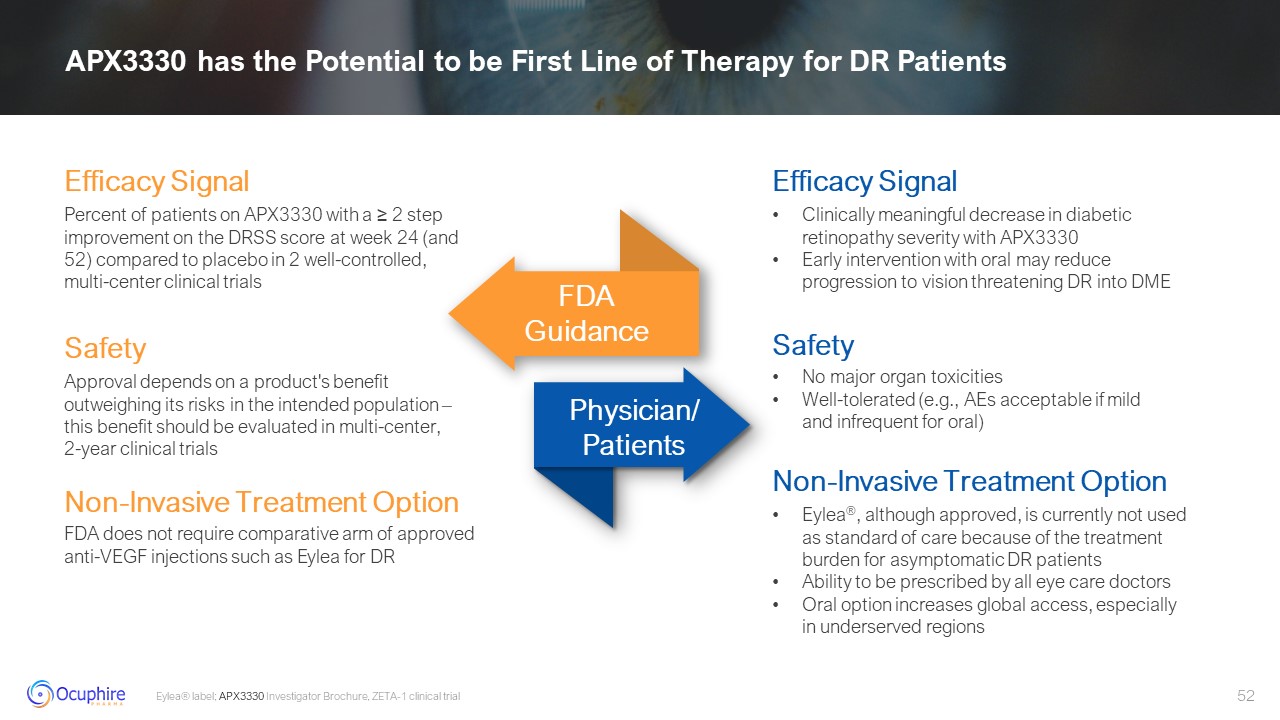
APX3330 has the Potential to be First Line of Therapy for DR Patients Eylea®
label; APX3330 Investigator Brochure, ZETA-1 clinical trial Clinically meaningful decrease in diabetic retinopathy severity with APX3330 Early intervention with oral may reduce progression to vision threatening DR into DME Efficacy
Signal Percent of patients on APX3330 with a ≥ 2 step improvement on the DRSS score at week 24 (and 52) compared to placebo in 2 well-controlled, multi-center clinical trials Efficacy Signal FDA
Guidance Physician/Patients Safety Non-Invasive Treatment Option Safety Approval depends on a product's benefit outweighing its risks in the intended population – this benefit should be evaluated in multi-center, 2-year clinical trials
FDA does not require comparative arm of approved anti-VEGF injections such as Eylea for DR Non-Invasive Treatment Option No major organ toxicities Well-tolerated (e.g., AEs acceptable if mild and infrequent for oral) Eylea®, although
approved, is currently not used as standard of care because of the treatment burden for asymptomatic DR patients Ability to be prescribed by all eye care doctors Oral option increases global access, especially in underserved regions
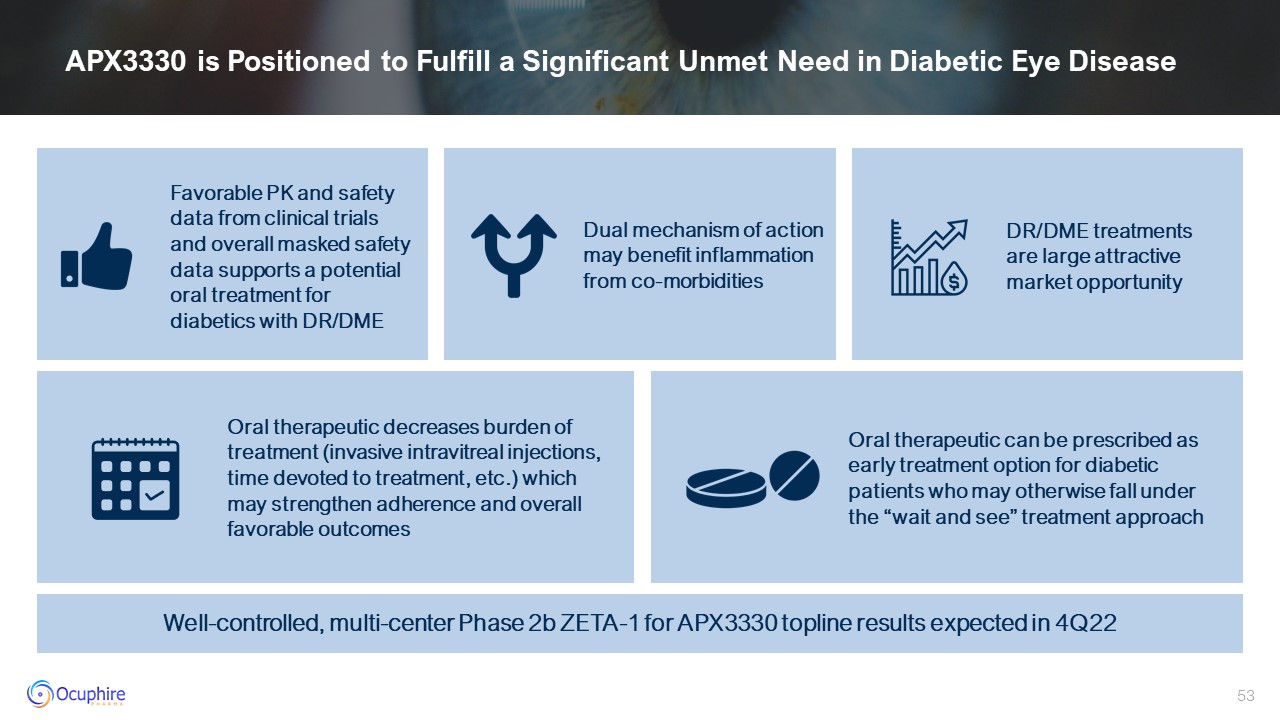
APX3330 is Positioned to Fulfill a Significant Unmet Need in Diabetic Eye
Disease Favorable PK and safety data from clinical trials and overall masked safety data supports a potential oral treatment for diabetics with DR/DME Dual mechanism of action may benefit inflammation from co-morbidities DR/DME treatments
are large attractive market opportunity Oral therapeutic can be prescribed as early treatment option for diabetic patients who may otherwise fall under the “wait and see” treatment approach Oral therapeutic decreases burden of treatment
(invasive intravitreal injections, time devoted to treatment, etc.) which may strengthen adherence and overall favorable outcomes Well-controlled, multi-center Phase 2b ZETA-1 for APX3330 topline results expected in 4Q22