EXHIBIT 99.1
Published on March 27, 2024

O c u p h i r e I n v e s t o r P r e s e n t a t i o n M a r c h 2 0 2
4 Exhibit 99.1

This presentation contains forward-looking statements within the meaning of the
Private Securities Litigation Reform Act of 1995. Such statements include, but are not limited to, statements concerning the End-of-Phase 2 meeting with the FDA to align on late-stage registration endpoints and study parameters, the launching
of RYZUMVI, the continued development of PS and LDP, our partnership with Viatris, the strength of our cash position, and the potential of APX3330 as an oral treatment for patients with non-proliferative diabetic retinopathy. These
forward-looking statements relate to us, our business prospects and our results of operations and are subject to certain risks and uncertainties posed by many factors and events that could cause our actual business, prospects and results of
operations to differ materially from those anticipated by such forward-looking statements. Factors that could cause or contribute to such differences include, but are not limited to, those described under the heading “Risk Factors” included
in our Annual Report on Form 10-K. Readers are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date of this presentation. In some cases, you can identify forward-looking statements by the
following words: “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “ongoing,” “plan,” “potential,” “predict,” “project,” “should,” “will,” “would” or the negative of these terms or other comparable
terminology, although not all forward-looking statements contain these words. We undertake no obligation to revise any forward-looking statements in order to reflect events or circumstances that might subsequently arise. These forward-looking
statements are based upon Ocuphire’s current expectations and involve assumptions that may never materialize or may prove to be incorrect. Actual results and the timing of events could differ materially from those anticipated in such
forward-looking statements as a result of various risks and uncertainties, including, without limitation: the success and timing of regulatory submissions and pre-clinical and clinical trials, including enrollment and data readouts;
regulatory requirements or developments; changes to or unanticipated events in connection with clinical trial designs and regulatory pathways; delays or difficulties in the enrollment of patients in clinical trials; substantial competition
and rapid technological change; our development of sales and marketing infrastructure; future revenue losses and profitability; our relatively short operating history; changes in capital resource requirements; risks related to the inability
of Ocuphire to obtain sufficient additional capital to continue to advance its product candidates and its preclinical programs; domestic and worldwide legislative, regulatory, political and economic developments; employee misconduct; changes
in market opportunities and acceptance; reliance on third-parties; future, potential product liability and securities litigation; system failures, unplanned events, or cyber incidents; the substantial number of shares subject to potential
issuance associated with our Equity Line of Credit arrangement with LPC; risks that our partnership with Viatris, or our other licensing arrangements, may not facilitate the commercialization or market acceptance of Ocuphire’s product
candidates; future fluctuations in the market price of our common stock; the success and timing of commercialization of any of Ocuphire’s product candidates; and obtaining and maintaining Ocuphire’s intellectual property rights. The
foregoing review of important factors that could cause actual events to differ from expectations should not be construed as exhaustive. Readers are urged to carefully review and consider the various disclosures made by us in this presentation
and in our reports filed with the SEC that advise interested parties of the risks and factors that may affect our business. All forward-looking statements contained in this presentation speak only as of the date on which they were made.
Ocuphire undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made. 2 Disclosures and Forward-Looking Statements
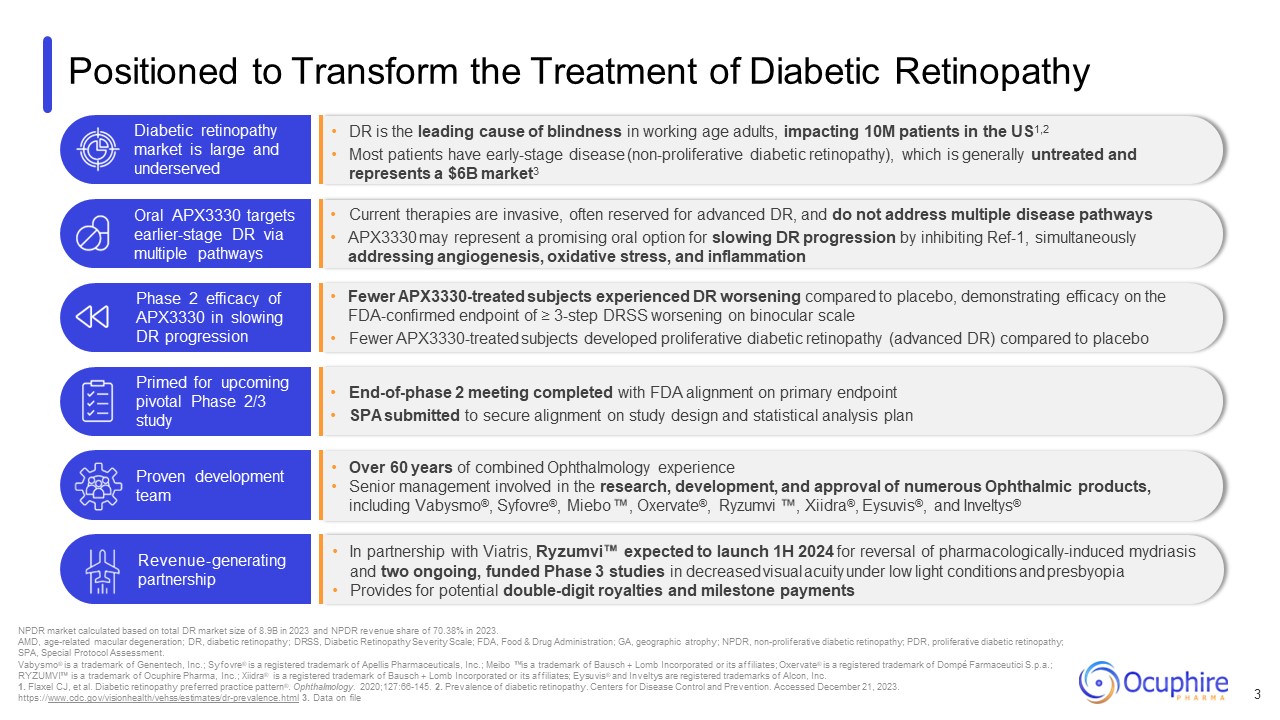
Positioned to Transform the Treatment of Diabetic Retinopathy 3 NPDR market
calculated based on total DR market size of 8.9B in 2023 and NPDR revenue share of 70.38% in 2023. AMD, age-related macular degeneration; DR, diabetic retinopathy; DRSS, Diabetic Retinopathy Severity Scale; FDA, Food & Drug
Administration; GA, geographic atrophy; NPDR, non-proliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy; SPA, Special Protocol Assessment. Vabysmo® is a trademark of Genentech, Inc.; Syfovre® is a registered trademark
of Apellis Pharmaceuticals, Inc.; Meibo is a trademark of Bausch + Lomb Incorporated or its affiliates; Oxervate® is a registered trademark of Dompé Farmaceutici S.p.a.; RYZUMVI is a trademark of Ocuphire Pharma, Inc.; Xiidra® is a registered
trademark of Bausch + Lomb Incorporated or its affiliates; Eysuvis® and Inveltys are registered trademarks of Alcon, Inc. 1. Flaxel CJ, et al. Diabetic retinopathy preferred practice pattern®. Ophthalmology. 2020;127:66-145. 2. Prevalence of
diabetic retinopathy. Centers for Disease Control and Prevention. Accessed December 21, 2023. https://www.cdc.gov/visionhealth/vehss/estimates/dr-prevalence.html 3. Data on file DR is the leading cause of blindness in working age adults,
impacting 10M patients in the US1,2 Most patients have early-stage disease (non-proliferative diabetic retinopathy), which is generally untreated and represents a $6B market3 Diabetic retinopathy market is large and underserved Current
therapies are invasive, often reserved for advanced DR, and do not address multiple disease pathways APX3330 may represent a promising oral option for slowing DR progression by inhibiting Ref-1, simultaneously addressing angiogenesis,
oxidative stress, and inflammation Oral APX3330 targets earlier-stage DR via multiple pathways Fewer APX3330-treated subjects experienced DR worsening compared to placebo, demonstrating efficacy on the FDA-confirmed endpoint of ≥ 3-step
DRSS worsening on binocular scale Fewer APX3330-treated subjects developed proliferative diabetic retinopathy (advanced DR) compared to placebo Phase 2 efficacy of APX3330 in slowing DR progression End-of-phase 2 meeting completed with FDA
alignment on primary endpoint SPA submitted to secure alignment on study design and statistical analysis plan Primed for upcoming pivotal Phase 2/3 study Over 60 years of combined Ophthalmology experience Senior management involved in the
research, development, and approval of numerous Ophthalmic products, including Vabysmo®, Syfovre®, Miebo , Oxervate®, Ryzumvi , Xiidra®, Eysuvis®, and Inveltys® In partnership with Viatris, Ryzumvi expected to launch 1H 2024 for reversal of
pharmacologically-induced mydriasis and two ongoing, funded Phase 3 studies in decreased visual acuity under low light conditions and presbyopia Provides for potential double-digit royalties and milestone payments Proven development
team Revenue-generating partnership
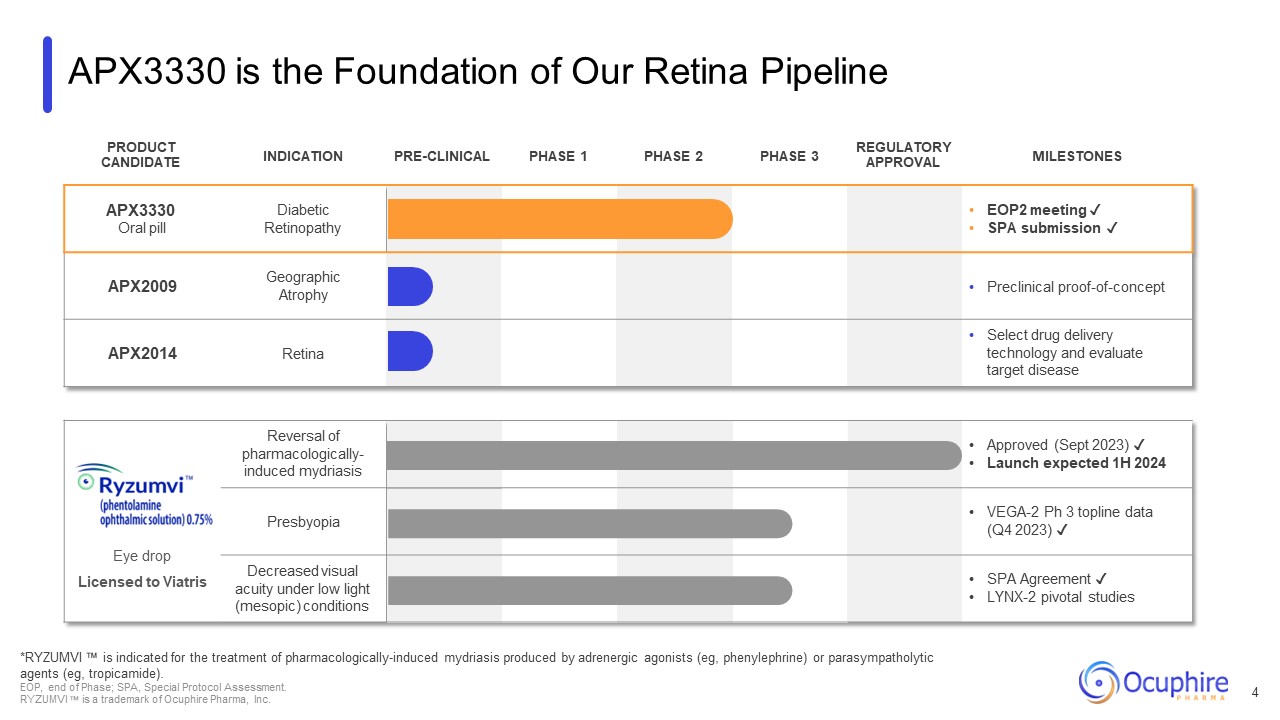
APX3330 is the Foundation of Our Retina Pipeline PRODUCT
CANDIDATE INDICATION PRE-CLINICAL PHASE 1 PHASE 2 PHASE 3 REGULATORY APPROVAL MILESTONES APX3330 Oral pill Diabetic Retinopathy EOP2 meeting ✓ SPA submission ✓ APX2009 Geographic Atrophy Preclinical
proof-of-concept APX2014 Retina Select drug delivery technology and evaluate target disease Eye drop Licensed to Viatris Reversal of pharmacologically- induced mydriasis Approved (Sept 2023) ✓ Launch expected 1H
2024 Presbyopia VEGA-2 Ph 3 topline data (Q4 2023) ✓ Decreased visual acuity under low light (mesopic) conditions SPA Agreement ✓ LYNX-2 pivotal studies *RYZUMVI is indicated for the treatment of pharmacologically-induced mydriasis
produced by adrenergic agonists (eg, phenylephrine) or parasympatholytic agents (eg, tropicamide). EOP, end of Phase; SPA, Special Protocol Assessment. RYZUMVI is a trademark of Ocuphire Pharma, Inc. 4
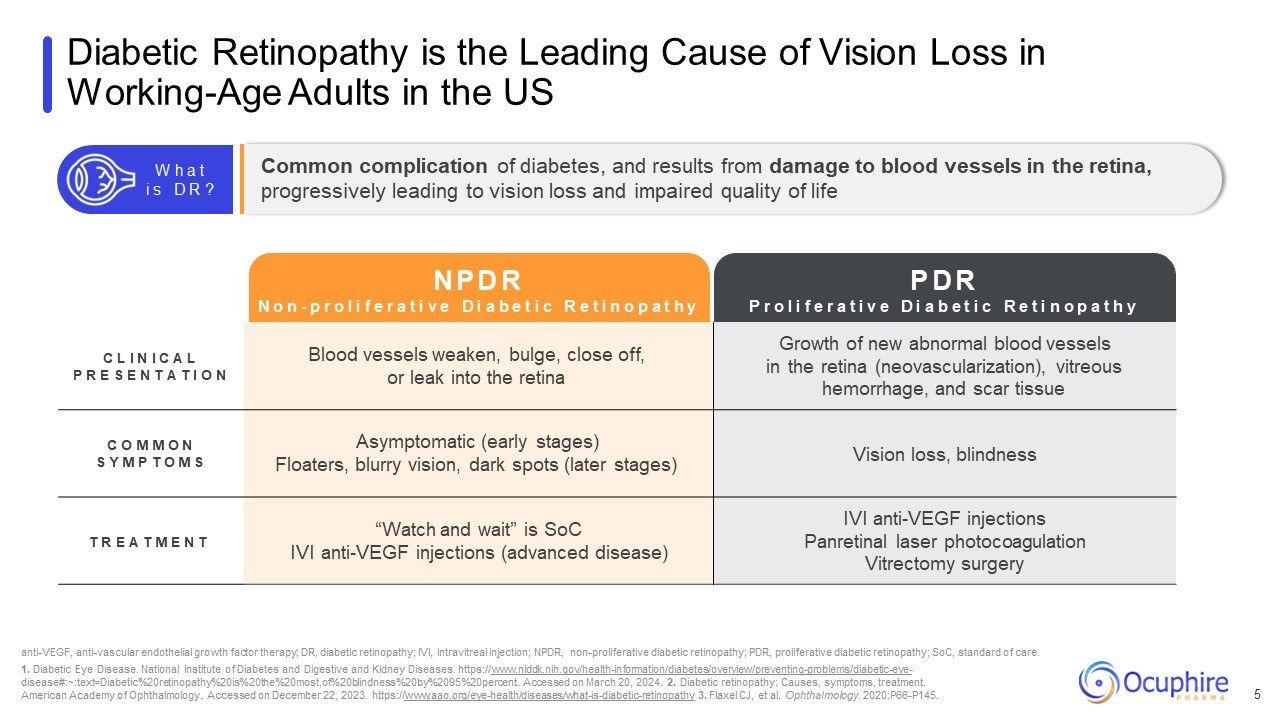
Diabetic Retinopathy is the Leading Cause of Vision Loss in Working-Age Adults
in the US 5 C L I N I C A L P R E S E N T A T I O N Blood vessels weaken, bulge, close off, or leak into the retina Growth of new abnormal blood vessels in the retina (neovascularization), vitreous hemorrhage, and scar tissue C O M M O
N S Y M P T O M S Asymptomatic (early stages) Floaters, blurry vision, dark spots (later stages) Vision loss, blindness T R E A T M E N T “Watch and wait” is SoC IVI anti-VEGF injections (advanced disease) IVI anti-VEGF injections
Panretinal laser photocoagulation Vitrectomy surgery anti-VEGF, anti-vascular endothelial growth factor therapy; DR, diabetic retinopathy; IVI, intravitreal injection; NPDR, non-proliferative diabetic retinopathy; PDR, proliferative diabetic
retinopathy; SoC, standard of care. 1. Diabetic Eye Disease. National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/diabetes/overview/preventing-problems/diabetic-eye-
disease#:~:text=Diabetic%20retinopathy%20is%20the%20most,of%20blindness%20by%2095%20percent. Accessed on March 20, 2024. 2. Diabetic retinopathy: Causes, symptoms, treatment. American Academy of Ophthalmology. Accessed on December 22, 2023.
https://www.aao.org/eye-health/diseases/what-is-diabetic-retinopathy 3. Flaxel CJ, et al. Ophthalmology. 2020:P66-P145. Common complication of diabetes, and results from damage to blood vessels in the retina, progressively leading to vision
loss and impaired quality of life W h a t i s D R ? N P D R N o n - p r o l i f e r a t i v e D i a b e t i c R e t i n o p a t h y P r o l i f e r a t i v e P D R D i a b e t i c R e t i n o p a t h y

DR Progression Can Have a Significant Impact On Functional Vision NOTE: The
severity of vision loss varies between individuals with DR DR, diabetic retinopathy; NPDR, non-proliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy. 1. [No authors listed]. ETDRS report number 12. Ophthalmology.
1991;98:823-833. 2. Diabetic Eye Disease. National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/diabetes/overview/preventing-
problems/diabetic-eye-disease#:~:text=Diabetic%20retinopathy%20is%20the%20most,of%20blindness%20by%2095%20percent. Accessed on March 5, 2024. N P D R P D R M i n i m a l v i s u a l d i s r u p t i o n S i g n i f i c a n t v i s i o
n l o s s ~50% of patients with severe NPDR will progress to PDR in 1 year1 Treating diabetic retinopathy early can reduce the risk of blindness by 95%2 6
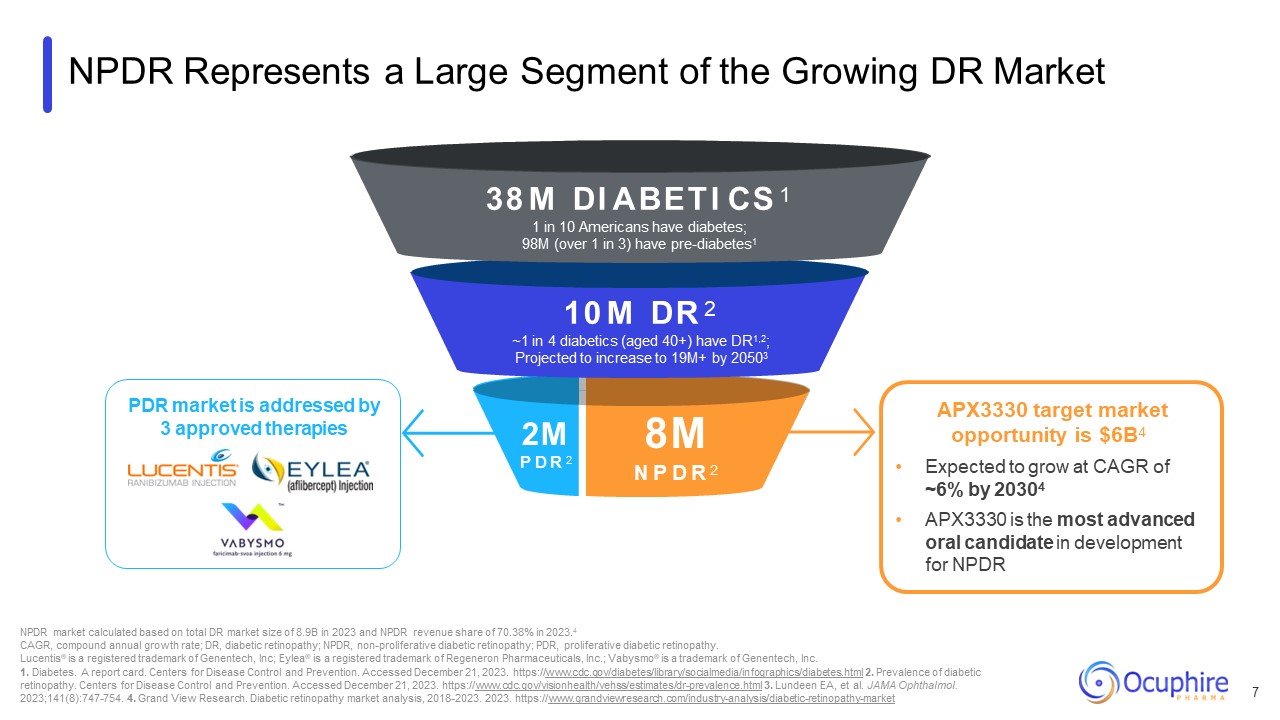
NPDR Represents a Large Segment of the Growing DR Market 2M P D R 2 38 M DI
ABETI CS 1 1 in 10 Americans have diabetes; 98M (over 1 in 3) have pre-diabetes1 10 M DR 2 ~1 in 4 diabetics (aged 40+) have DR1,2; Projected to increase to 19M+ by 20503 8M N P D R 2 NPDR market calculated based on total DR market
size of 8.9B in 2023 and NPDR revenue share of 70.38% in 2023.4 CAGR, compound annual growth rate; DR, diabetic retinopathy; NPDR, non-proliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy. Lucentis® is a registered
trademark of Genentech, Inc; Eylea® is a registered trademark of Regeneron Pharmaceuticals, Inc.; Vabysmo® is a trademark of Genentech, Inc. 1. Diabetes. A report card. Centers for Disease Control and Prevention. Accessed December 21, 2023.
https://www.cdc.gov/diabetes/library/socialmedia/infographics/diabetes.html 2. Prevalence of diabetic retinopathy. Centers for Disease Control and Prevention. Accessed December 21, 2023.
https://www.cdc.gov/visionhealth/vehss/estimates/dr-prevalence.html 3. Lundeen EA, et al. JAMA Ophthalmol. 2023;141(8):747-754. 4. Grand View Research. Diabetic retinopathy market analysis, 2018-2023. 2023.
https://www.grandviewresearch.com/industry-analysis/diabetic-retinopathy-market APX3330 target market opportunity is $6B4 Expected to grow at CAGR of ~6% by 20304 APX3330 is the most advanced oral candidate in development for
NPDR 7 PDR market is addressed by 3 approved therapies
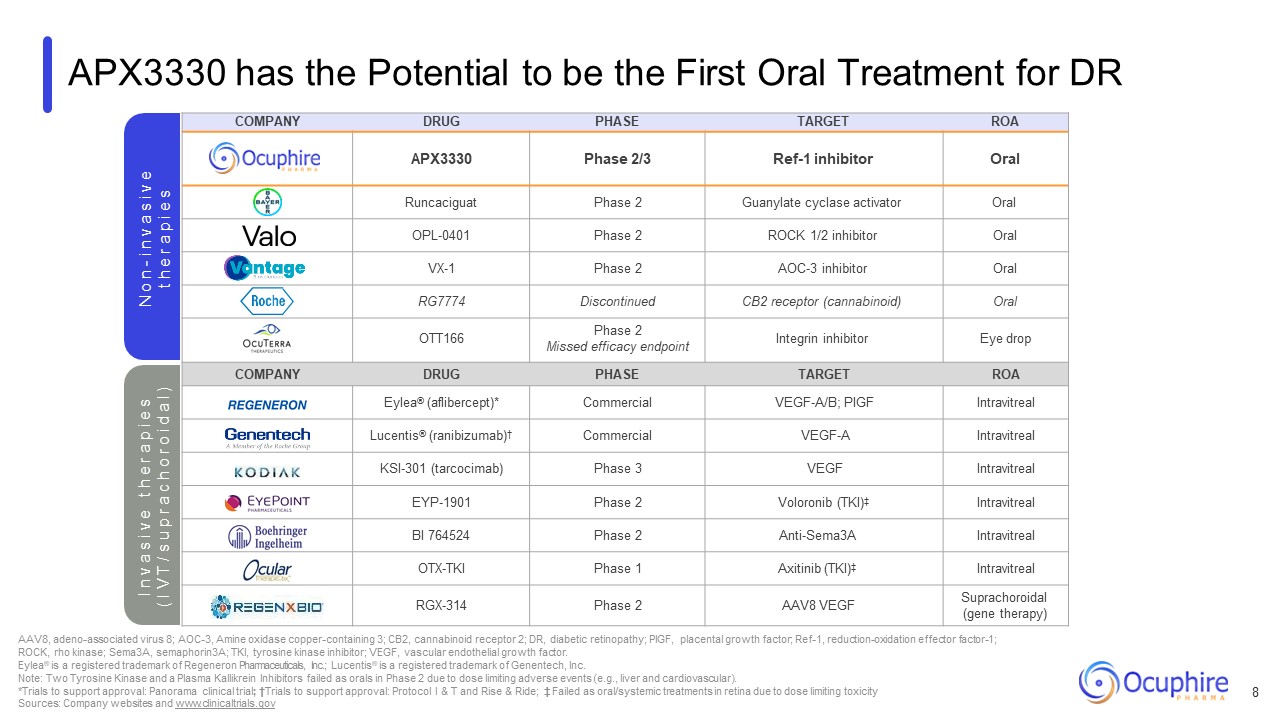
COMPANY DRUG PHASE TARGET ROA APX3330 Phase 2/3 Ref-1
inhibitor Oral Runcaciguat Phase 2 Guanylate cyclase activator Oral OPL-0401 Phase 2 ROCK 1/2 inhibitor Oral VX-1 Phase 2 AOC-3 inhibitor Oral RG7774 Discontinued CB2 receptor (cannabinoid) Oral OTT166 Phase 2 Missed
efficacy endpoint Integrin inhibitor Eye drop COMPANY DRUG PHASE TARGET ROA Eylea® (aflibercept)* Commercial VEGF-A/B; PIGF Intravitreal Lucentis® (ranibizumab)† Commercial VEGF-A Intravitreal KSI-301 (tarcocimab) Phase
3 VEGF Intravitreal EYP-1901 Phase 2 Voloronib (TKI)‡ Intravitreal BI 764524 Phase 2 Anti-Sema3A Intravitreal OTX-TKI Phase 1 Axitinib (TKI)‡ Intravitreal RGX-314 Phase 2 AAV8 VEGF Suprachoroidal (gene therapy) APX3330 has
the Potential to be the First Oral Treatment for DR N o n - i n v a s i v e t h e r a p i e s AAV8, adeno-associated virus 8; AOC-3, Amine oxidase copper-containing 3; CB2, cannabinoid receptor 2; DR, diabetic retinopathy; PIGF, placental
growth factor; Ref-1, reduction-oxidation effector factor-1; ROCK, rho kinase; Sema3A, semaphorin3A; TKI, tyrosine kinase inhibitor; VEGF, vascular endothelial growth factor. Eylea® is a registered trademark of Regeneron Pharmaceuticals,
Inc.; Lucentis® is a registered trademark of Genentech, Inc. Note: Two Tyrosine Kinase and a Plasma Kallikrein Inhibitors failed as orals in Phase 2 due to dose limiting adverse events (e.g., liver and cardiovascular). *Trials to support
approval: Panorama clinical trial; †Trials to support approval: Protocol I & T and Rise & Ride; ‡ Failed as oral/systemic treatments in retina due to dose limiting toxicity Sources: Company websites and www.clinicaltrials.gov I n v a
s i v e t h e r a p i e s ( I V T / s u p r a c h o r o i d a l ) 8

APX3330 The most advanced oral program currently in development for diabetic
retinopathy
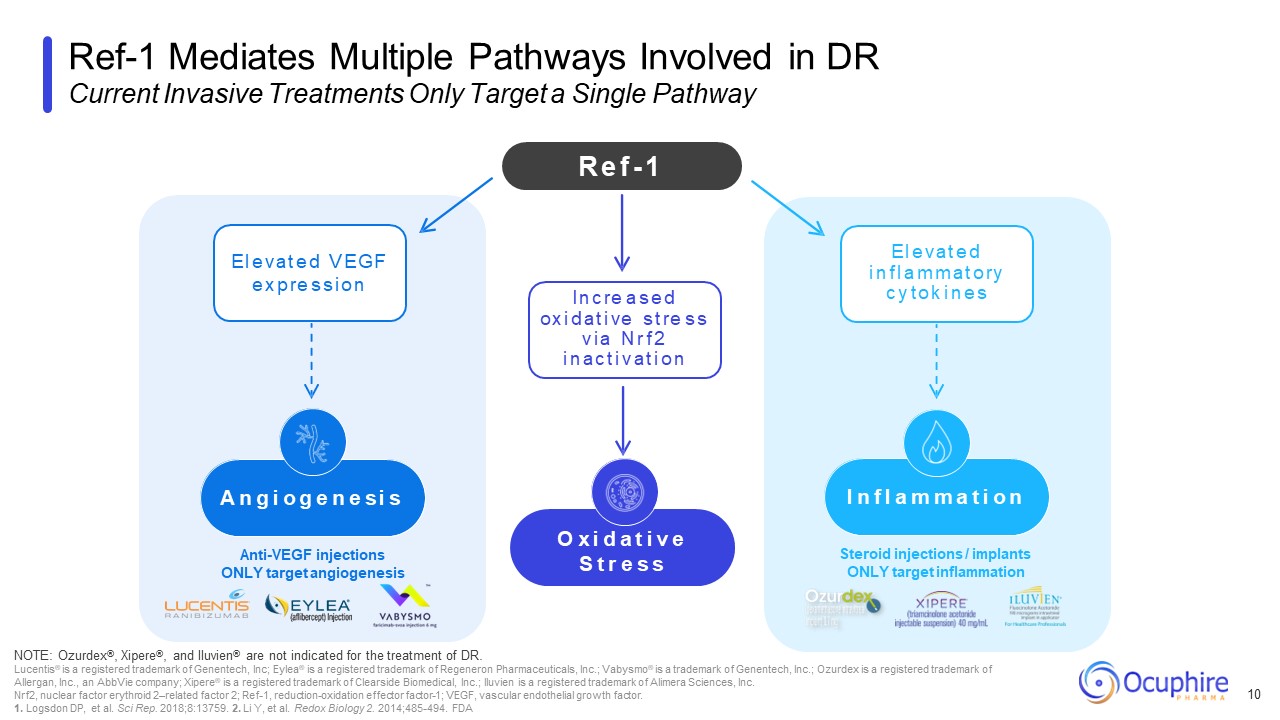
Steroid injections / implants ONLY target inflammation Anti-VEGF injections
ONLY target angiogenesis Ref-1 Mediates Multiple Pathways Involved in DR Current Invasive Treatments Only Target a Single Pathway 10 NOTE: Ozurdex®, Xipere®, and Iluvien® are not indicated for the treatment of DR. Lucentis® is a
registered trademark of Genentech, Inc; Eylea® is a registered trademark of Regeneron Pharmaceuticals, Inc.; Vabysmo® is a trademark of Genentech, Inc.; Ozurdex is a registered trademark of Allergan, Inc., an AbbVie company; Xipere® is a
registered trademark of Clearside Biomedical, Inc.; Iluvien is a registered trademark of Alimera Sciences, Inc. Nrf2, nuclear factor erythroid 2–related factor 2; Ref-1, reduction-oxidation effector factor-1; VEGF, vascular endothelial
growth factor. 1. Logsdon DP, et al. Sci Rep. 2018;8:13759. 2. Li Y, et al. Redox Biology 2. 2014;485-494. FDA R e f - 1 O x i d a t i v e S t r e s s I n c re a s e d ox i d a t i ve s t re s s v i a N r f 2 i n a c t i v a t i o n E
l e v a t e d V E G F e x p re s s i o n A n g i o g e n e s i s I n f l a m m a t i o n E l e v a t e d i n f l a m m a t o ry c y t o k i n e s
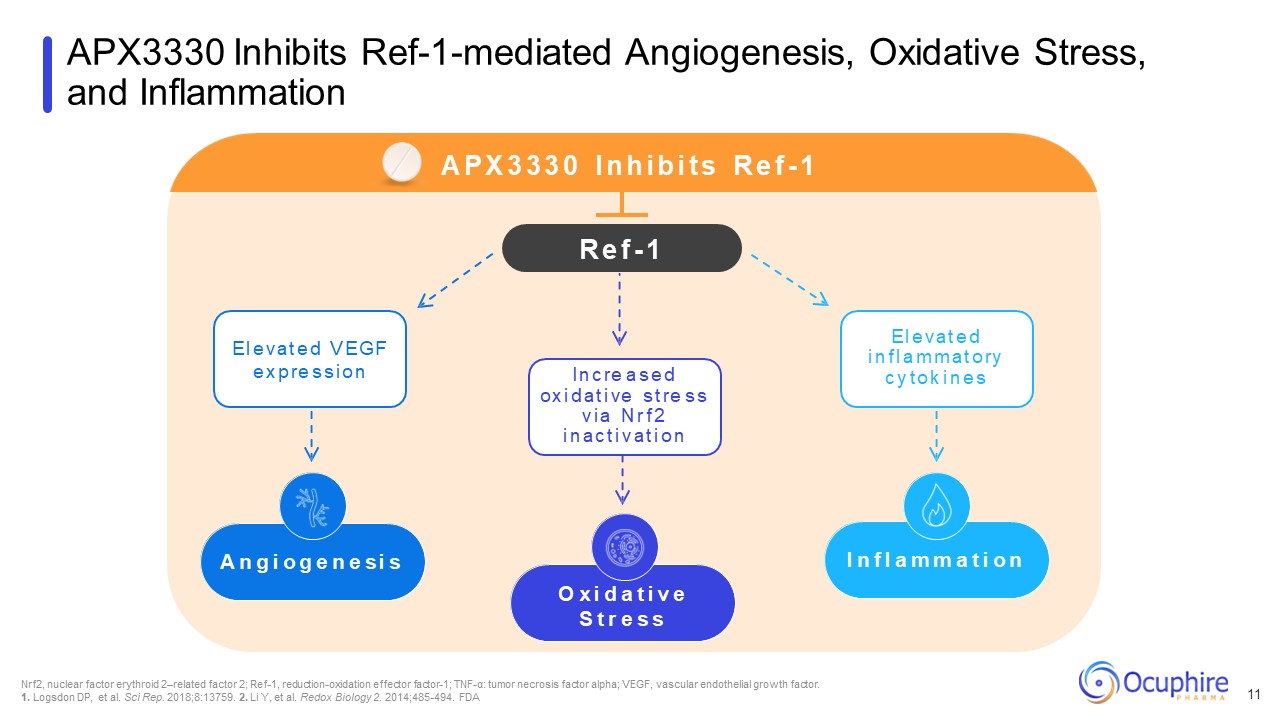
APX3330 Inhibits Ref-1-mediated Angiogenesis, Oxidative Stress, and
Inflammation Nrf2, nuclear factor erythroid 2–related factor 2; Ref-1, reduction-oxidation effector factor-1; TNF-α: tumor necrosis factor alpha; VEGF, vascular endothelial growth factor. 1. Logsdon DP, et al. Sci Rep. 2018;8:13759. 2. Li
Y, et al. Redox Biology 2. 2014;485-494. FDA O x i d a t i v e S t r e s s A n g i o g e n e s i s I n f l a m m a t i o n A P X 3 3 3 0 I n h i b i t s R e f - 1 R e f - 1 I n c re a s e d ox i d a t i ve s t re s s v i a N r f 2 i
n a c t i v a t i o n E l e v a t e d V E G F e x p re s s i o n E l e v a t e d i n f l a m m a t o ry c y t o k i n e s 11
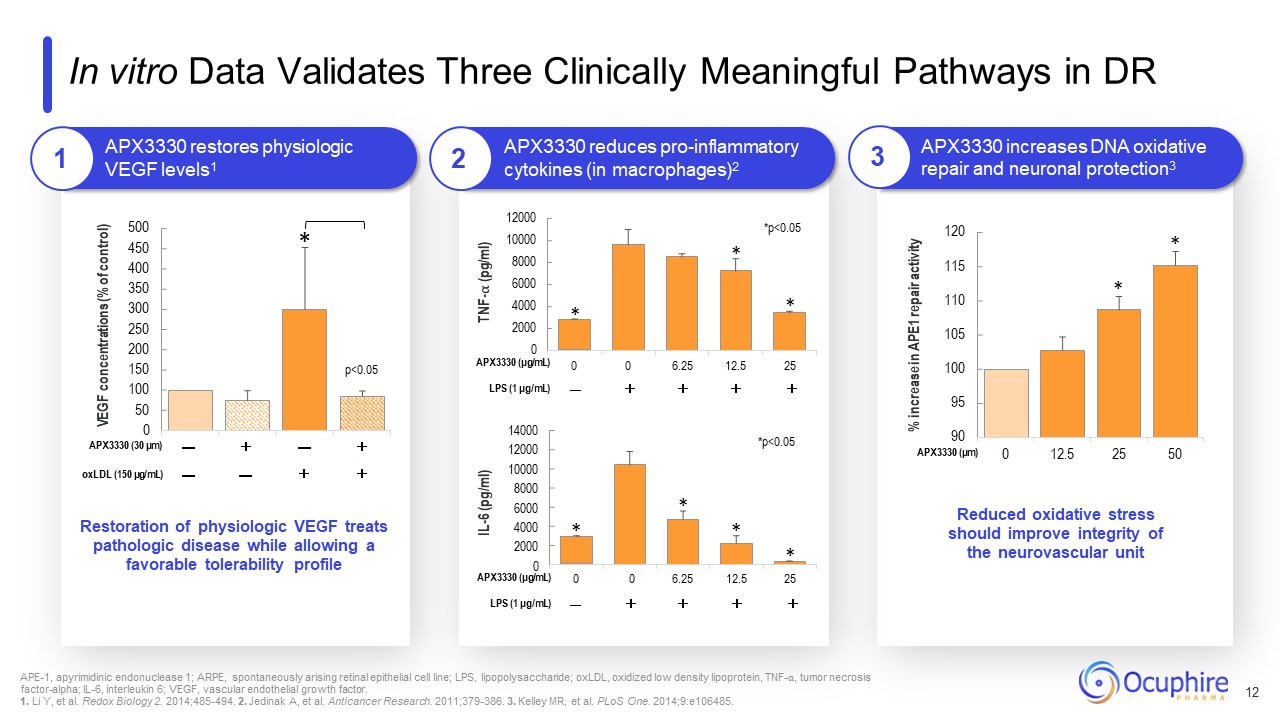
95 100 105 110 115 120 0 12.5 25 50 500 450 400 350 300 250 200 150 100 50 0 VEGF
concentrations (% of control) * p<0.05 APX3330 restores physiologic VEGF levels1 APX3330 (30 μm) — + — + oxLDL (150 μg/mL) — — + + TNF-⍺ (pg/ml) IL-6 (pg/ml) * * * *p<0.05 *p<0.05 * % increase in APE1 repair
activity * Restoration of physiologic VEGF treats pathologic disease while allowing a favorable tolerability profile Reduced oxidative stress should improve integrity of the neurovascular unit * * * In vitro Data Validates Three
Clinically Meaningful Pathways in DR APX3330 reduces pro-inflammatory cytokines (in macrophages)2 APX3330 increases DNA oxidative repair and neuronal
protection3 14000 12000 10000 8000 6000 4000 2000 0 12000 10000 8000 6000 4000 2000 0 APX3330 (μg/mL) 0 0 6.25 12.5 25 LPS (1 μg/mL) — + + + + 90 APX3330 (μm) APX3330 (μg/mL) 0 0 6.25 12.5 25 LPS (1
μg/mL) — + + + + * APE-1, apyrimidinic endonuclease 1; ARPE, spontaneously arising retinal epithelial cell line; LPS, lipopolysaccharide; oxLDL, oxidized low density lipoprotein, TNF-⍺, tumor necrosis factor-alpha; IL-6, interleukin 6;
VEGF, vascular endothelial growth factor. 1. Li Y, et al. Redox Biology 2. 2014;485-494. 2. Jedinak A, et al. Anticancer Research. 2011;379-386. 3. Kelley MR, et al. PLoS One. 2014;9:e106485. 12 1 2 3

ZETA-1 Clinical Trial A Phase 2 Randomized, Placebo-Controlled, Double-Masked
Study of APX3330 in DR is Complete
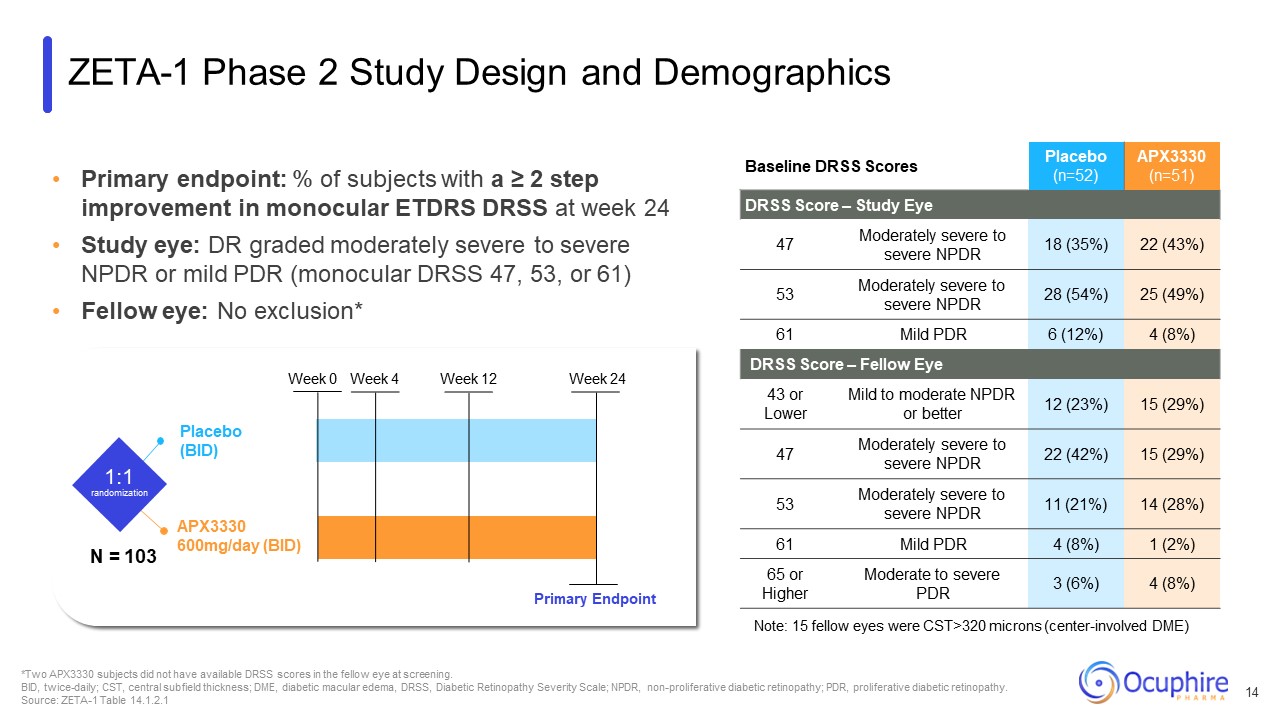
Primary endpoint: % of subjects with a ≥ 2 step improvement in monocular ETDRS
DRSS at week 24 Study eye: DR graded moderately severe to severe NPDR or mild PDR (monocular DRSS 47, 53, or 61) Fellow eye: No exclusion* Baseline DRSS Scores Placebo (n=52) APX3330 (n=51) DRSS Score – Study Eye 47 Moderately
severe to severe NPDR 18 (35%) 22 (43%) 53 Moderately severe to severe NPDR 28 (54%) 25 (49%) 61 Mild PDR 6 (12%) 4 (8%) DRSS Score – Fellow Eye 43 or Lower Mild to moderate NPDR or better 12 (23%) 15 (29%) 47 Moderately
severe to severe NPDR 22 (42%) 15 (29%) 53 Moderately severe to severe NPDR 11 (21%) 14 (28%) 61 Mild PDR 4 (8%) 1 (2%) 65 or Higher Moderate to severe PDR 3 (6%) 4 (8%) Note: 15 fellow eyes were CST>320 microns
(center-involved DME) *Two APX3330 subjects did not have available DRSS scores in the fellow eye at screening. BID, twice-daily; CST, central subfield thickness; DME, diabetic macular edema, DRSS, Diabetic Retinopathy Severity Scale; NPDR,
non-proliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy. Source: ZETA-1 Table 14.1.2.1 APX3330 600mg/day (BID) Placebo (BID) 1:1 randomization Week 0 Week 4 Week 12 Week 24 Primary Endpoint ZETA-1 Phase 2
Study Design and Demographics N = 103 14
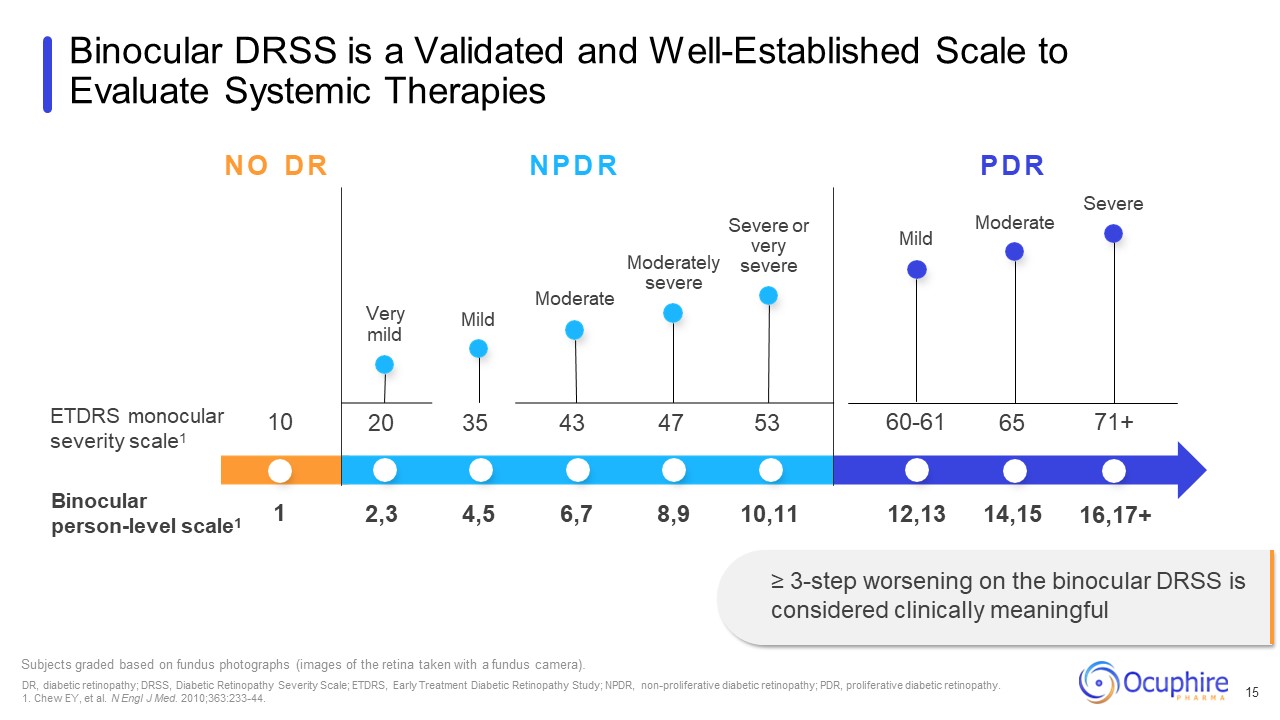
ETDRS monocular severity scale1 Binocular person-level scale1 Moderately
severe Severe or very severe Mild Subjects graded based on fundus photographs (images of the retina taken with a fundus camera). DR, diabetic retinopathy; DRSS, Diabetic Retinopathy Severity Scale; ETDRS, Early Treatment Diabetic
Retinopathy Study; NPDR, non-proliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy. 1. Chew EY, et al. N Engl J Med. 2010;363:233-44. Binocular DRSS is a Validated and Well-Established Scale to Evaluate Systemic
Therapies 15 Moderate N O D R N P D R P D R Severe Very mild Moderate Mild 10 20 35 43 47 53 60-61 65 71+ 1 2,3 4,5 6,7 8,9 10,11 12,13 14,15 16,17+ ≥ 3-step worsening on the binocular DRSS is considered clinically
meaningful
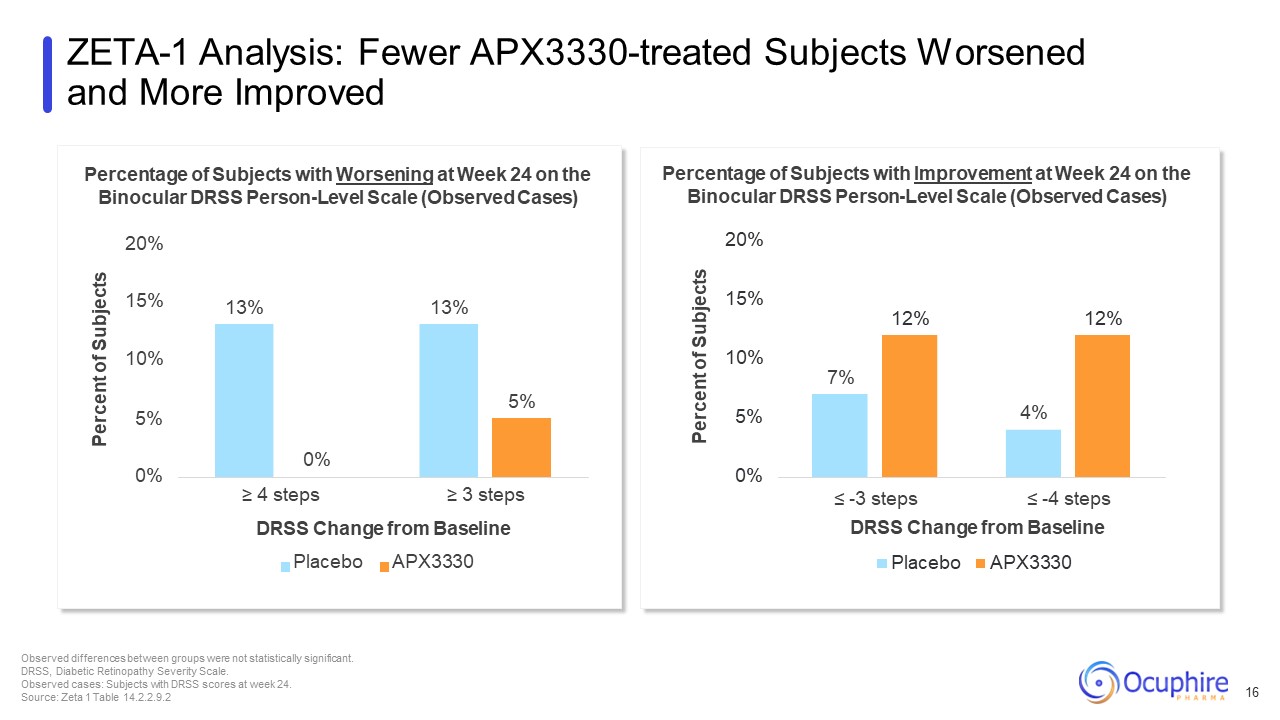
7% 4% 12% 12% 0% 5% 10% 15% Percent of Subjects ≤ -3 steps ≤ -4
steps DRSS Change from Baseline Placebo APX3330 Percentage of Subjects with Improvement at Week 24 on the Binocular DRSS Person-Level Scale (Observed Cases) 20% 13% 13% 5% 0% 10% 5% 15% Percent of Subjects 0% ≥ 4 steps ≥ 3 steps
DRSS Change from Baseline Placebo APX3330 Percentage of Subjects with Worsening at Week 24 on the Binocular DRSS Person-Level Scale (Observed Cases) 20% ZETA-1 Analysis: Fewer APX3330-treated Subjects Worsened and More Improved Observed
differences between groups were not statistically significant. DRSS, Diabetic Retinopathy Severity Scale. Observed cases: Subjects with DRSS scores at week 24. Source: Zeta 1 Table 14.2.2.9.2 16
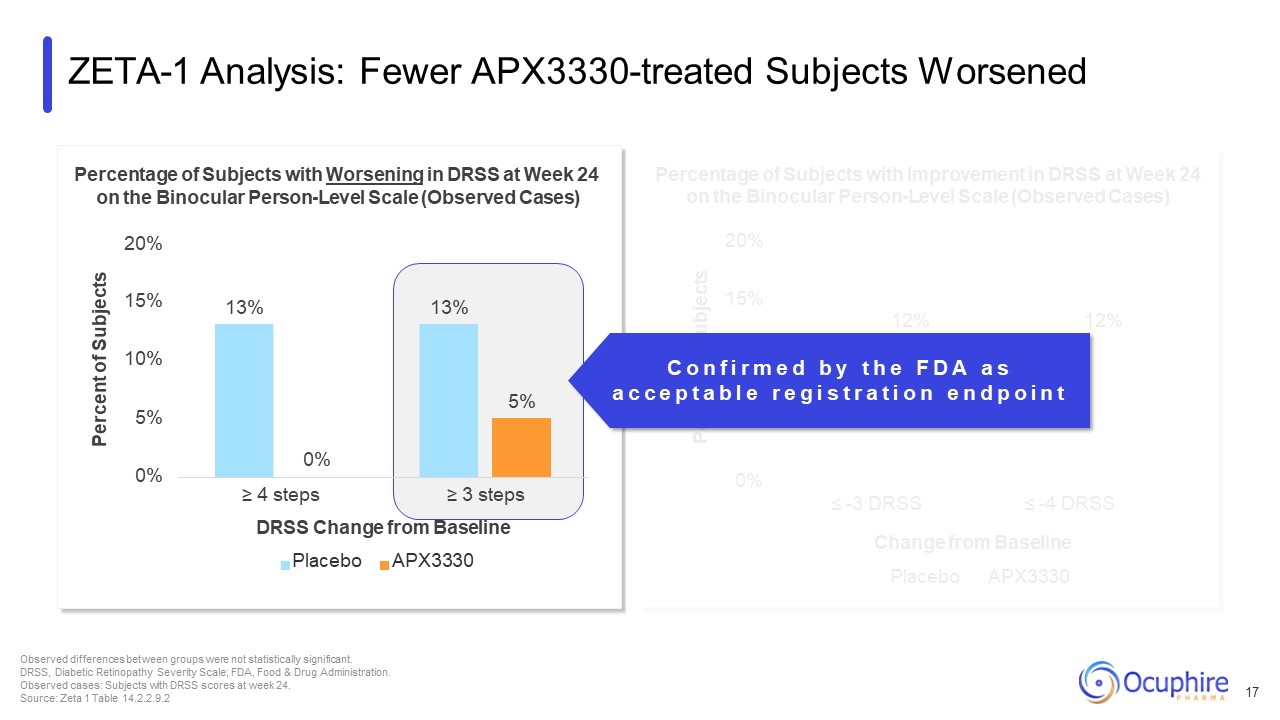
20% 15% 12% 12% 10% 7% 5% 4% 0% ≤ -3 DRSS ≤ -4 DRSS Percent of
Subjects Percentage of Subjects with Improvement in DRSS at Week 24 on the Binocular Person-Level Scale (Observed Cases) Change from Baseline Placebo APX3330 ZETA-1 Analysis: Fewer APX3330-treated Subjects Worsened 17 Observed
differences between groups were not statistically significant. DRSS, Diabetic Retinopathy Severity Scale; FDA, Food & Drug Administration. Observed cases: Subjects with DRSS scores at week 24. Source: Zeta 1 Table
14.2.2.9.2 13% 13% 5% 0% 5% 10% 15% Percentage of Subjects with Worsening in DRSS at Week 24 on the Binocular Person-Level Scale (Observed Cases) 20% Percent of Subjects 0% ≥ 4 steps ≥ 3 steps DRSS Change from Baseline Placebo
APX3330 C o n f i r m e d b y t h e F D A a s a c c e p t a b l e r e g i s t r a t i o n e n d p o i n t
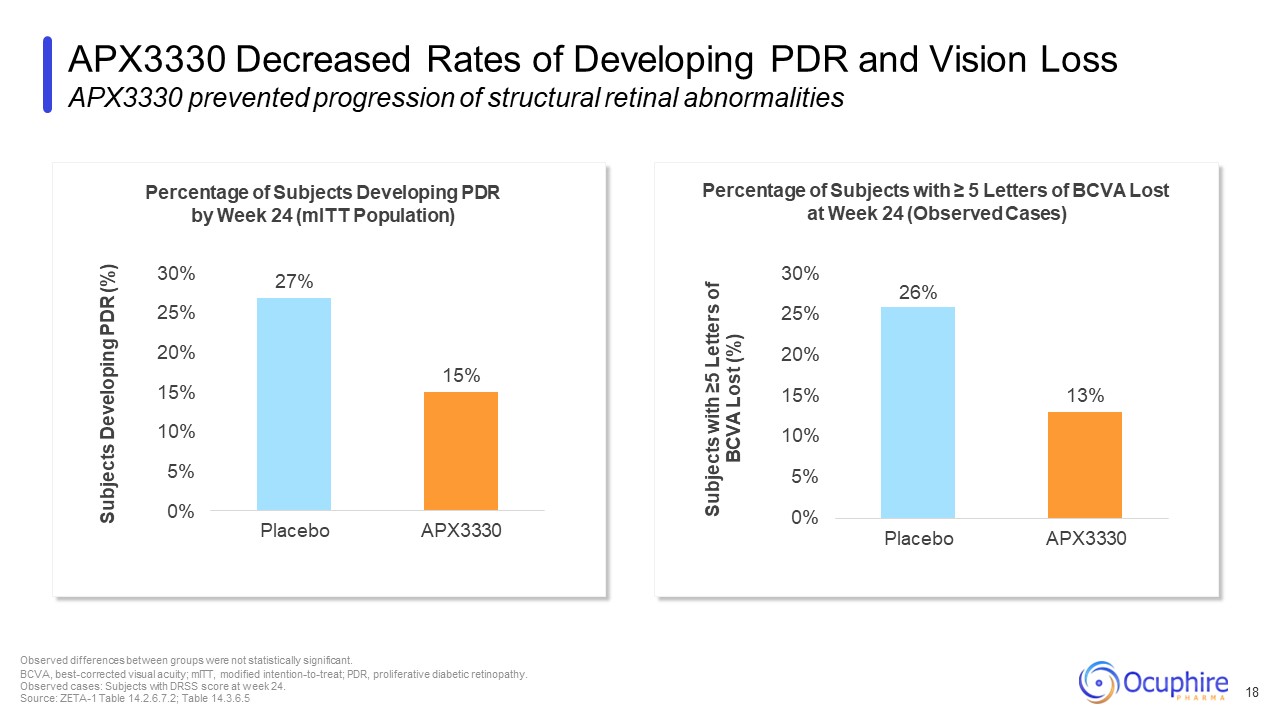
Percentage of Subjects Developing PDR by Week 24 (mITT Population) Percentage
of Subjects with ≥ 5 Letters of BCVA Lost at Week 24 (Observed Cases) 26% APX3330 Decreased Rates of Developing PDR and Vision Loss APX3330 prevented progression of structural retinal abnormalities Observed differences between groups were
not statistically significant. BCVA, best-corrected visual acuity; mITT, modified intention-to-treat; PDR, proliferative diabetic retinopathy. Observed cases: Subjects with DRSS score at week 24. Source: ZETA-1 Table 14.2.6.7.2; Table
14.3.6.5 18 27% 15% 25% 20% 15% 10% 5% 0% 30% Placebo APX3330 Subjects Developing PDR (%) 13% 10% 5% 0% 25% 20% 15% 30% Placebo APX3330 Subjects with ≥5 Letters of BCVA Lost (%)
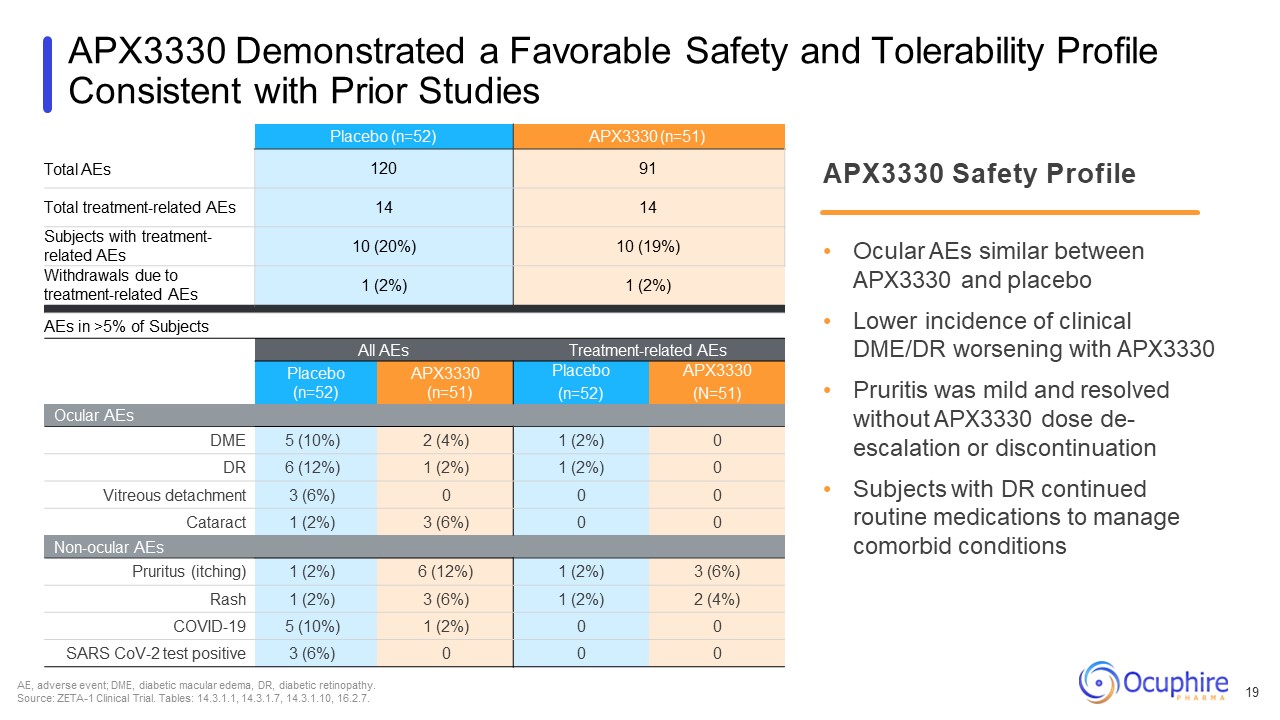
APX3330 Demonstrated a Favorable Safety and Tolerability Profile Consistent with
Prior Studies Total AEs Placebo (n=52) APX3330 (n=51) 120 91 Total treatment-related AEs 14 14 Subjects with treatment- related AEs 10 (20%) 10 (19%) Withdrawals due to treatment-related AEs 1 (2%) 1 (2%) AEs in >5% of
Subjects All AEs Treatment-related AEs Placebo (n=52) APX3330 (n=51) Placebo (n=52) APX3330 (N=51) Ocular AEs DME 5 (10%) 2 (4%) 1 (2%) 0 DR 6 (12%) 1 (2%) 1 (2%) 0 Vitreous detachment 3 (6%) 0 0 0 Cataract 1 (2%) 3
(6%) 0 0 Non-ocular AEs Pruritus (itching) 1 (2%) 6 (12%) 1 (2%) 3 (6%) Rash 1 (2%) 3 (6%) 1 (2%) 2 (4%) COVID-19 5 (10%) 1 (2%) 0 0 SARS CoV-2 test positive 3 (6%) 0 0 0 APX3330 Safety Profile Ocular AEs similar
between APX3330 and placebo Lower incidence of clinical DME/DR worsening with APX3330 Pruritis was mild and resolved without APX3330 dose de- escalation or discontinuation Subjects with DR continued routine medications to manage comorbid
conditions AE, adverse event; DME, diabetic macular edema, DR, diabetic retinopathy. Source: ZETA-1 Clinical Trial. Tables: 14.3.1.1, 14.3.1.7, 14.3.1.10, 16.2.7. 19

ZETA-2 Clinical Trial A Phase 2/3 Randomized,
Placebo-Controlled, Double-Masked Study of APX3330 in NPDR is Planned
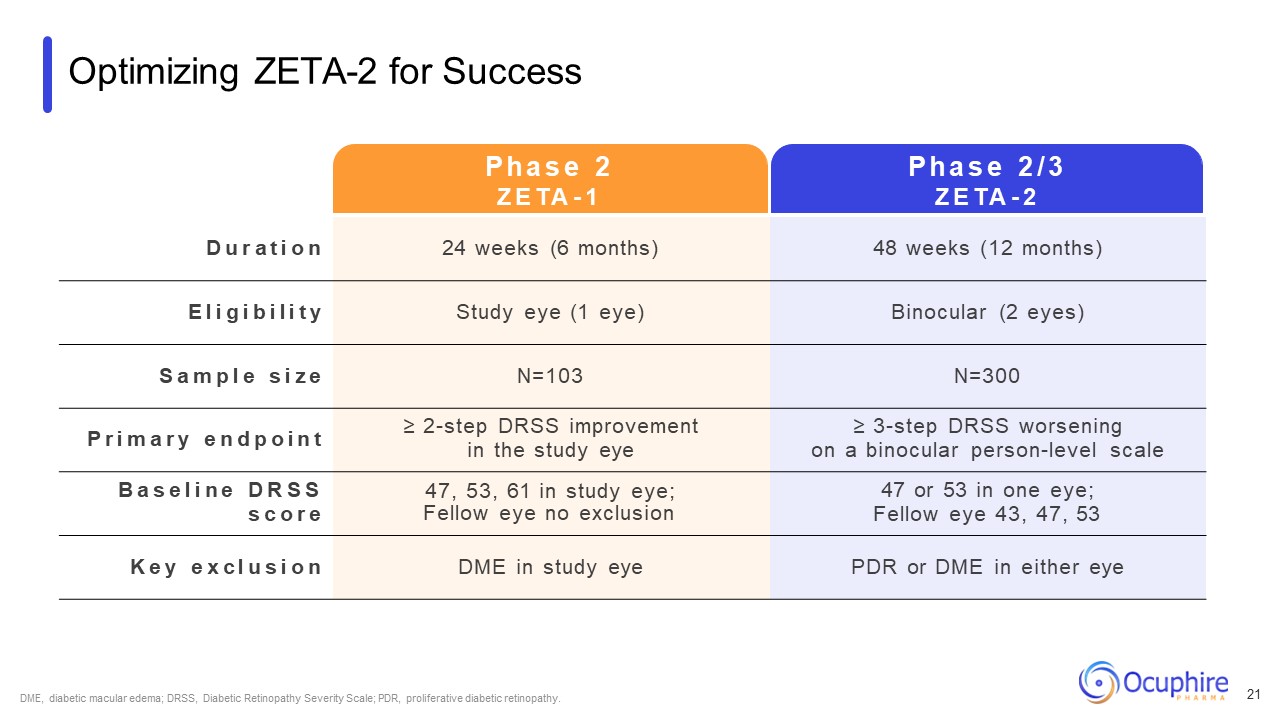
Optimizing ZETA-2 for Success D u r a t i o n 24 weeks (6 months) 48 weeks
(12 months) E l i g i b i l i t y Study eye (1 eye) Binocular (2 eyes) S a m p l e s i z e N=103 N=300 P r i m a r y e n d p o i n t ≥ 2-step DRSS improvement in the study eye ≥ 3-step DRSS worsening on a binocular person-level
scale B a s e l i n e D R S S s c o r e 47, 53, 61 in study eye; Fellow eye no exclusion 47 or 53 in one eye; Fellow eye 43, 47, 53 K e y e x c l u s i o n DME in study eye PDR or DME in either eye P h a s e 2 Z E TA - 1 P h a s e
2 / 3 Z E TA - 2 DME, diabetic macular edema; DRSS, Diabetic Retinopathy Severity Scale; PDR, proliferative diabetic retinopathy. 21
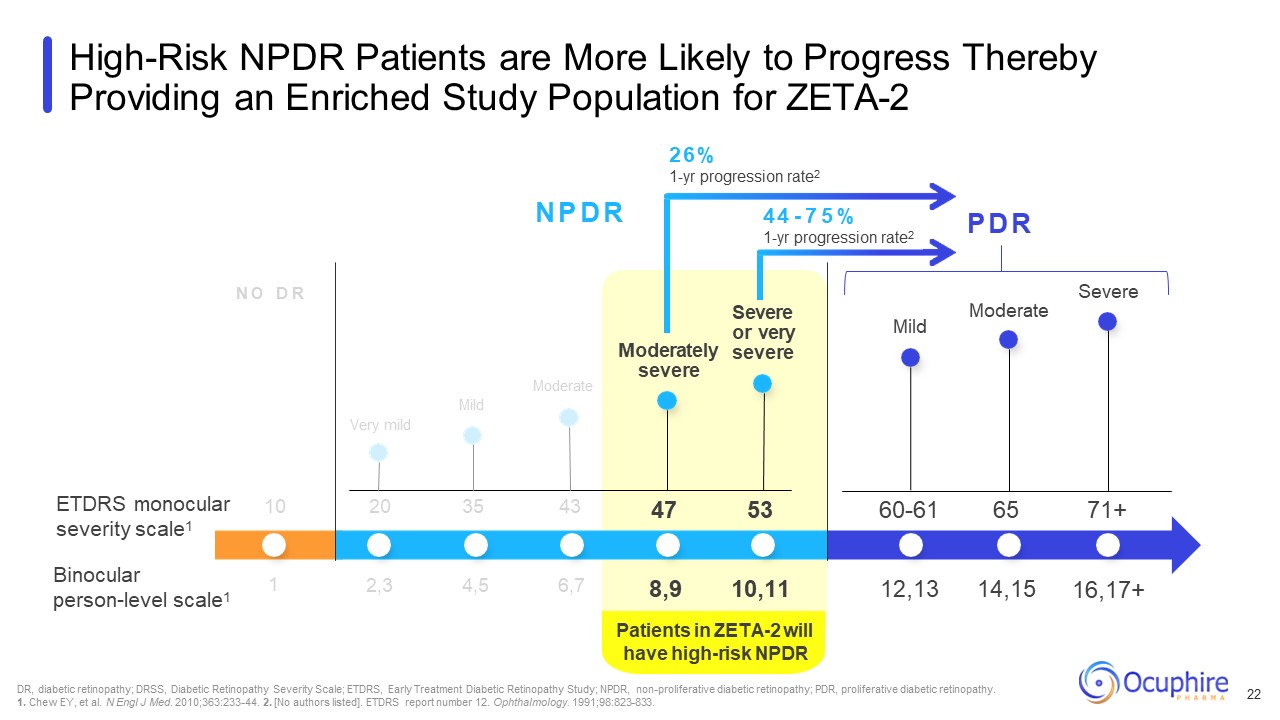
Patients in ZETA-2 will have high-risk NPDR N O D R N P D R P D R ETDRS
monocular severity scale1 Binocular person-level scale1 Very mild Moderate Mild Moderately severe Severe or very severe Mild DR, diabetic retinopathy; DRSS, Diabetic Retinopathy Severity Scale; ETDRS, Early Treatment Diabetic
Retinopathy Study; NPDR, non-proliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy. 1. Chew EY, et al. N Engl J Med. 2010;363:233-44. 2. [No authors listed]. ETDRS report number 12. Ophthalmology.
1991;98:823-833. High-Risk NPDR Patients are More Likely to Progress Thereby Providing an Enriched Study Population for ZETA-2 10 20 35 43 Moderate Severe 2,3 4,5 6,7 47 53 60-61 65 71+ 8,9 10,11 12,13 14,15 16,17+ 1 26%
1-yr progression rate2 44 - 7 5 % 1-yr progression rate2 22
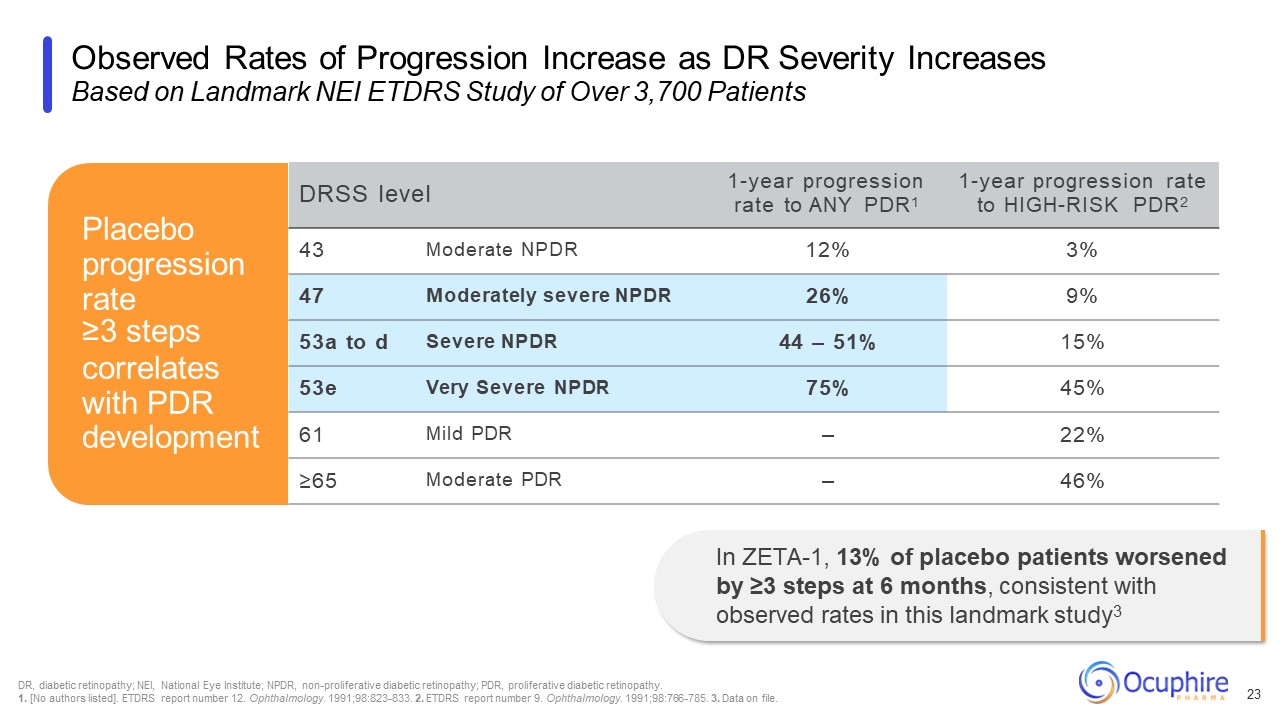
Observed Rates of Progression Increase as DR Severity Increases Based on
Landmark NEI ETDRS Study of Over 3,700 Patients 23 DRSS level 1-year progression rate to ANY PDR1 1-year progression rate to HIGH-RISK PDR2 43 Moderate NPDR 12% 3% 47 Moderately severe NPDR 26% 9% 53a to d Severe NPDR 44 –
51% 15% 53e Very Severe NPDR 75% 45% 61 Mild PDR – 22% ≥65 Moderate PDR – 46% Placebo progression rate ≥3 steps correlates with PDR development In ZETA-1, 13% of placebo patients worsened by ≥3 steps at 6 months, consistent
with observed rates in this landmark study3 DR, diabetic retinopathy; NEI, National Eye Institute; NPDR, non-proliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy. 1. [No authors listed]. ETDRS report number 12.
Ophthalmology. 1991;98:823-833. 2. ETDRS report number 9. Ophthalmology. 1991;98:766-785. 3. Data on file.
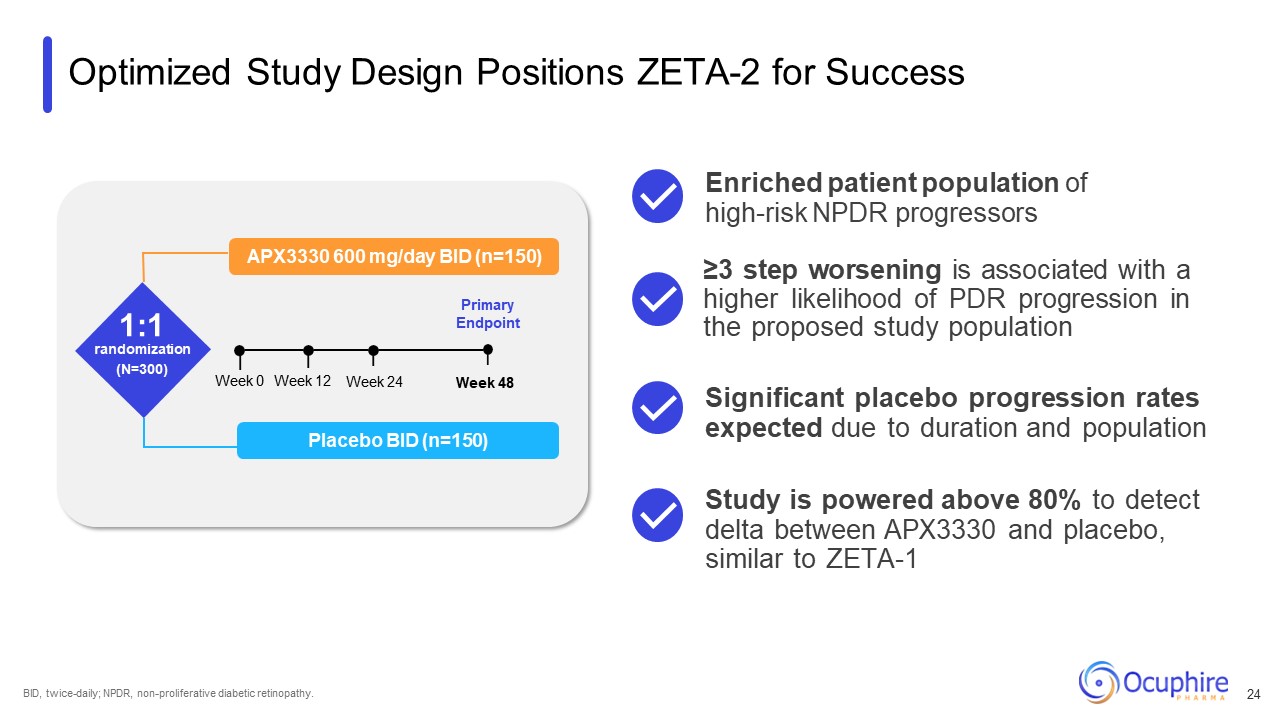
Optimized Study Design Positions ZETA-2 for Success APX3330 600 mg/day BID
(n=150) 1:1 randomization (N=300) Primary Endpoint Placebo BID (n=150) Week 0 Week 24 Week 12 Week 48 BID, twice-daily; NPDR, non-proliferative diabetic retinopathy. ≥3 step worsening is associated with a higher likelihood of PDR
progression in the proposed study population Significant placebo progression rates expected due to duration and population Enriched patient population of high-risk NPDR progressors Study is powered above 80% to detect delta between APX3330
and placebo, similar to ZETA-1 24

Jeff Heier, MD Ophthalmic Consultants of Boston “If ZETA-1 results are
repeated in Phase 3, I would place virtually all of my diabetic patients on oral APX3330 and treat locally only as needed." "Ref-1 biology targets three pillars of diabetic eye disease: angiogenesis, inflammation, and oxidative stress. This
is promising in the quest to provide non-invasive, early options for patients." Peter Kaiser, MD Cleveland Clinic “I enjoy working with the team to develop an innovative protocol design to enroll the patients most likely to have
progressive disease while keeping the study practical to help enrollment." Arshad Khanani, MD Sierra Eye Associates Globally Recognized Retina Specialists Support APX3330 Development 25

Partnership with Viatris Ryzumvi (phentolomine ophthalmic solution)
0.75% RYZUMVI is a trademark of Ocuphire Pharma, Inc.
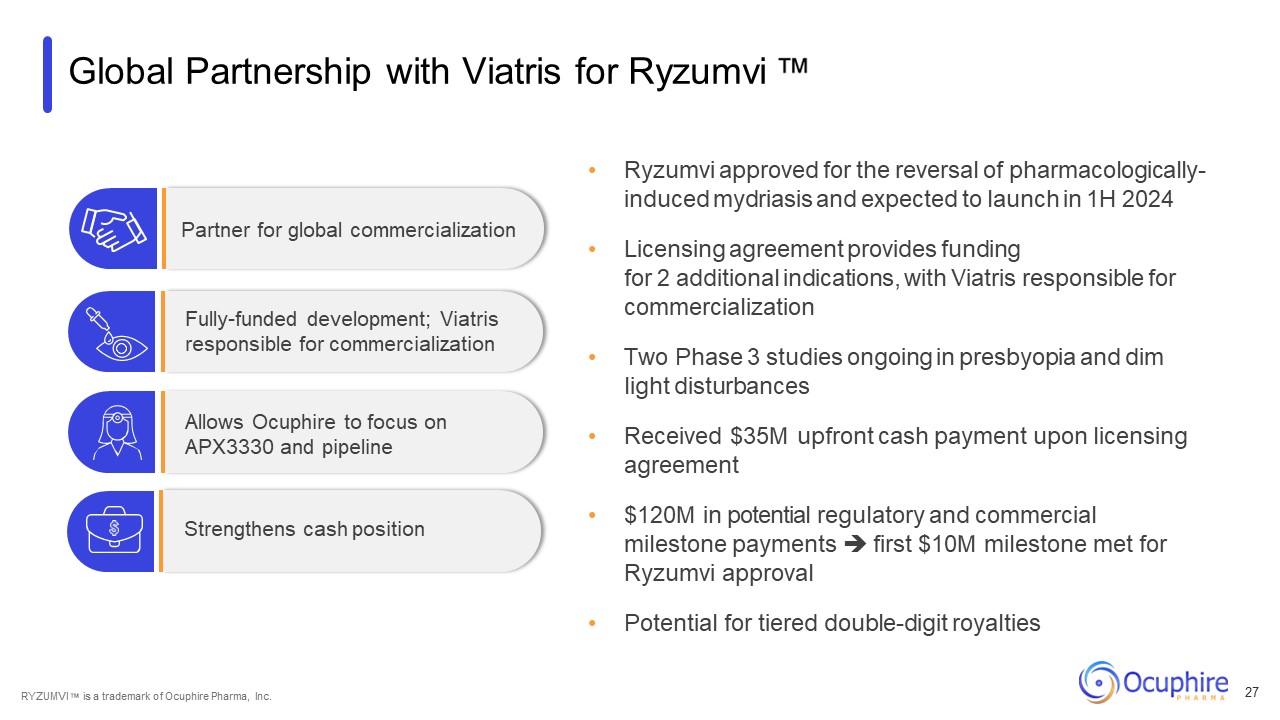
Global Partnership with Viatris for Ryzumvi Ryzumvi approved for the reversal
of pharmacologically- induced mydriasis and expected to launch in 1H 2024 Licensing agreement provides funding for 2 additional indications, with Viatris responsible for commercialization Two Phase 3 studies ongoing in presbyopia and dim
light disturbances Received $35M upfront cash payment upon licensing agreement $120M in potential regulatory and commercial milestone payments first $10M milestone met for Ryzumvi approval Potential for tiered double-digit
royalties Partner for global commercialization Fully-funded development; Viatris responsible for commercialization Allows Ocuphire to focus on APX3330 and pipeline Strengthens cash position RYZUMVI is a trademark of Ocuphire Pharma,
Inc. 27
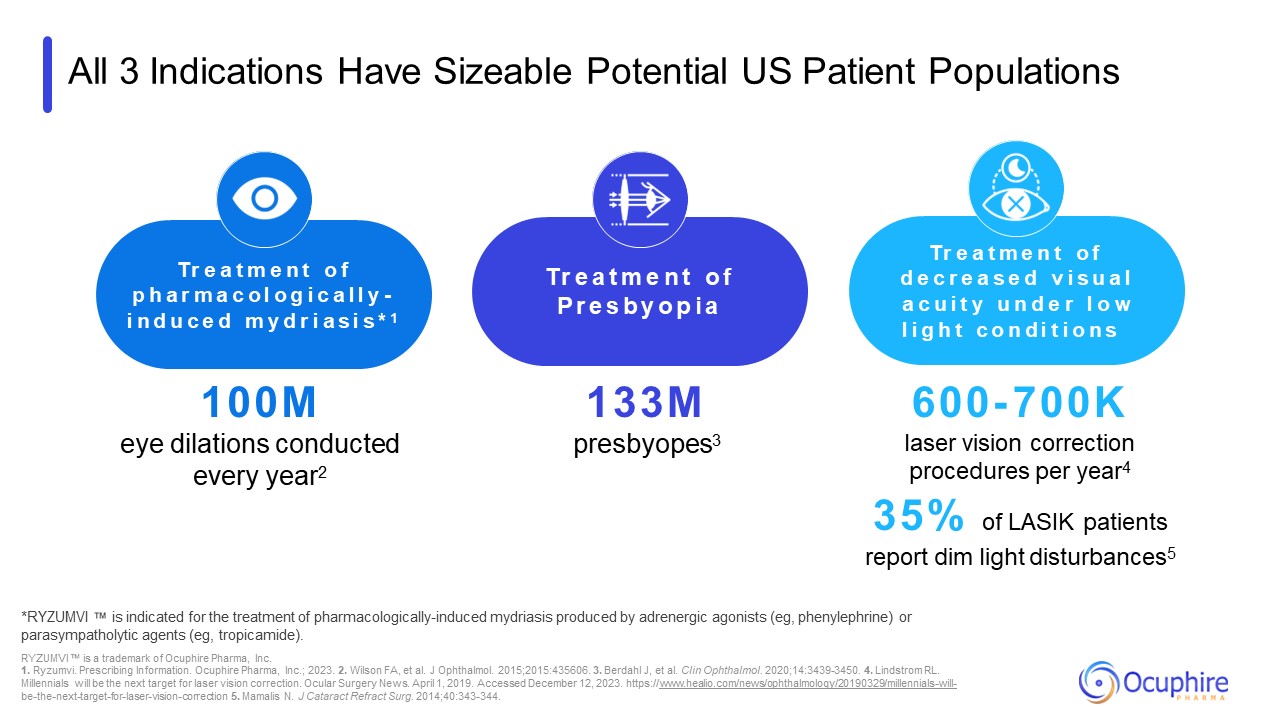
All 3 Indications Have Sizeable Potential US Patient Populations *RYZUMVI is
indicated for the treatment of pharmacologically-induced mydriasis produced by adrenergic agonists (eg, phenylephrine) or parasympatholytic agents (eg, tropicamide). RYZUMVI is a trademark of Ocuphire Pharma, Inc. 1. Ryzumvi. Prescribing
Information. Ocuphire Pharma, Inc.; 2023. 2. Wilson FA, et al. J Ophthalmol. 2015;2015:435606. 3. Berdahl J, et al. Clin Ophthalmol. 2020;14:3439-3450. 4. Lindstrom RL. Millennials will be the next target for laser vision correction. Ocular
Surgery News. April 1, 2019. Accessed December 12, 2023. https://www.healio.com/news/ophthalmology/20190329/millennials-will- be-the-next-target-for-laser-vision-correction 5. Mamalis N. J Cataract Refract Surg. 2014;40:343-344. 100M eye
dilations conducted every year2 Tr e a t m e n t o f p h a r m a c o l o g i c a l l y - i n d u c e d m y d r i a s i s * 1 Tr e a t m e n t o f P r e s b y o p i a Tr e a t m e n t o f d e c r e a s e d v i s u a l a c u i t y u n d e
r l o w l i g h t c o n d i t i o n s 133M presbyopes3 600- 700K laser vision correction procedures per year4 35% of LASIK patients report dim light disturbances5
Highly Experienced Team with Meaningful Expertise Over 60 years of proven
clinical, commercial, and transaction experience Involved in the research, development, and approval of numerous Ophthalmic products: Ophthalmic Experts 29 Ash Jayagopal, PhD, MBA Chief Scientific & Development Officer Vabysmo® is
a trademark of Genentech, Inc.; Syfovre® is a registered trademark of Apellis Pharmaceuticals, Inc.; RYZUMVI is a trademark of Ocuphire Pharma, Inc.; Eysuvis® and Inveltys are registered trademarks of Alcon, Inc.; Meibo is a trademark of
Bausch + Lomb Incorporated or its affiliates; Oxervate® is a registered trademark of Dompé Farmaceutici S.p.a.; Xiidra® is a registered trademark of Bausch + Lomb Incorporated or its affiliates; George Magrath, MD, MBA, MS Chief Executive
Officer Nirav Jhaveri, MBA Chief Financial Officer Joseph Schachle, MBA Chief Operating Officer
DR, diabetic retinopathy; FDA, Food and Drug Administration. Ocuphire is
Positioned to Transform the Treatment of Diabetic Retinopathy Extensive understanding of large, underserved DR market Addressing unmet needs by targeting multiple DR pathways with oral treatment Demonstrated efficacy in slowing DR
progression in completed Phase 2 study Primed for pivotal Phase 2/3 study with FDA-confirmed endpoint Proven development team with decades of Ophthalmic expertise Revenue-generating partnership strengthens cash position 30