EXHIBIT 99.1
Published on May 10, 2024

Exhibit 99.1

NPDR Subset Analysis of ZETA-1 Phase 2 Trial A moderate to severe
NPDR-qualifying subgroup analysis to inform future clinical trials NPDR, non-proliferative diabetic retinopathy.
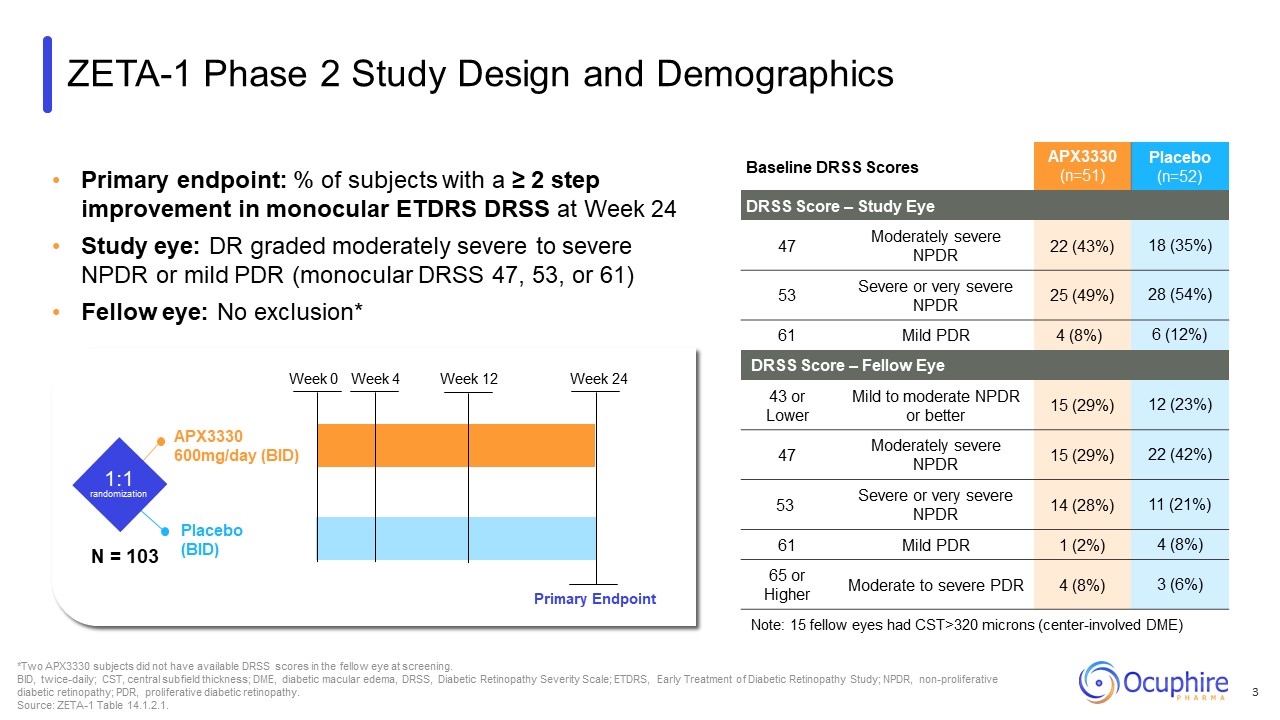
Primary endpoint: % of subjects with a ≥ 2 step improvement in monocular ETDRS
DRSS at Week 24 Study eye: DR graded moderately severe to severe NPDR or mild PDR (monocular DRSS 47, 53, or 61) Fellow eye: No exclusion* Baseline DRSS Scores APX3330 (n=51) Placebo (n=52) DRSS Score – Study Eye 47 Moderately
severe NPDR 22 (43%) 18 (35%) 53 Severe or very severe NPDR 25 (49%) 28 (54%) 61 Mild PDR 4 (8%) 6 (12%) DRSS Score – Fellow Eye 43 or Lower Mild to moderate NPDR or better 15 (29%) 12 (23%) 47 Moderately severe NPDR 15
(29%) 22 (42%) 53 Severe or very severe NPDR 14 (28%) 11 (21%) 61 Mild PDR 1 (2%) 4 (8%) 65 or Higher Moderate to severe PDR 4 (8%) 3 (6%) Note: 15 fellow eyes had CST>320 microns (center-involved DME) *Two APX3330 subjects
did not have available DRSS scores in the fellow eye at screening. BID, twice-daily; CST, central subfield thickness; DME, diabetic macular edema, DRSS, Diabetic Retinopathy Severity Scale; ETDRS, Early Treatment of Diabetic Retinopathy
Study; NPDR, non-proliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy. Source: ZETA-1 Table 14.1.2.1. APX3330 600mg/day (BID) Placebo (BID) 1:1 randomization Week 0 Week 4 Week 12 Week 24 Primary
Endpoint ZETA-1 Phase 2 Study Design and Demographics N = 103 3
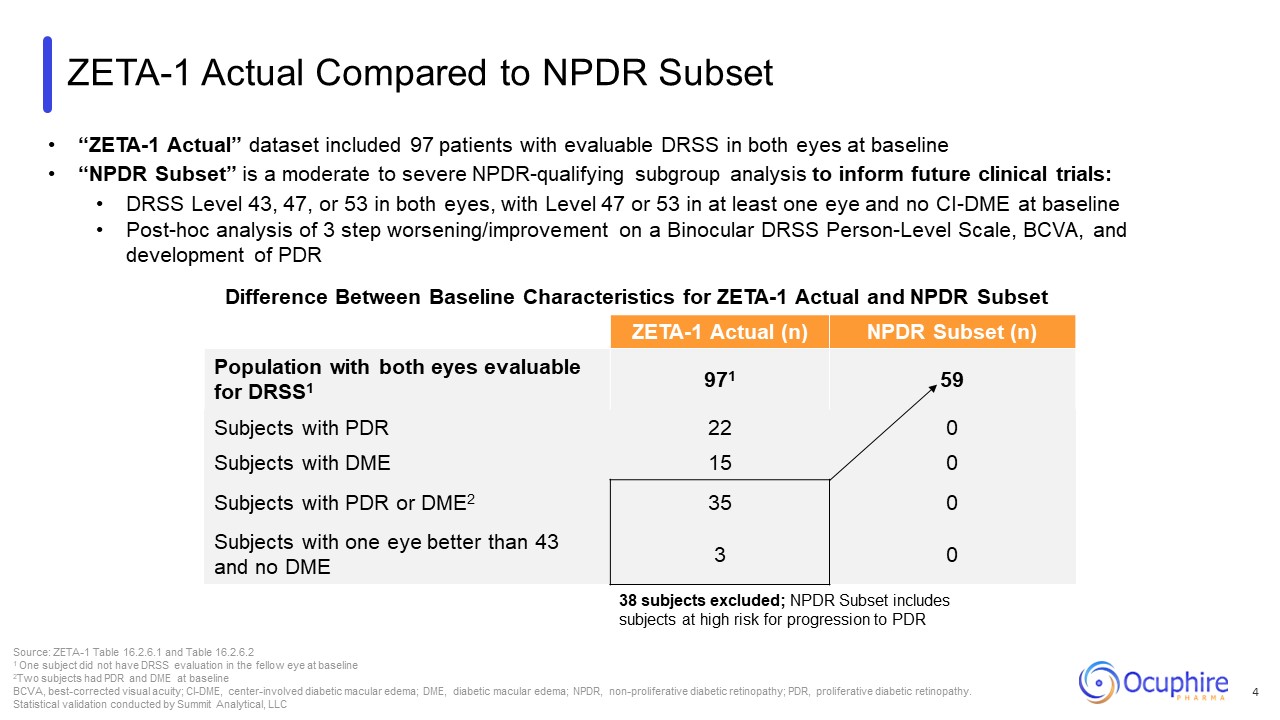
ZETA-1 Actual Compared to NPDR Subset Difference Between Baseline
Characteristics for ZETA-1 Actual and NPDR Subset ZETA-1 Actual (n) NPDR Subset (n) Population with both eyes evaluable for DRSS1 971 59 Subjects with PDR 22 0 Subjects with DME 15 0 Subjects with PDR or DME2 35 0 Subjects
with one eye better than 43 and no DME 3 0 Source: ZETA-1 Table 16.2.6.1 and Table 16.2.6.2 1 One subject did not have DRSS evaluation in the fellow eye at baseline 2Two subjects had PDR and DME at baseline BCVA, best-corrected visual
acuity; CI-DME, center-involved diabetic macular edema; DME, diabetic macular edema; NPDR, non-proliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy. Statistical validation conducted by Summit Analytical, LLC “ZETA-1
Actual” dataset included 97 patients with evaluable DRSS in both eyes at baseline “NPDR Subset” is a moderate to severe NPDR-qualifying subgroup analysis to inform future clinical trials: DRSS Level 43, 47, or 53 in both eyes, with Level 47
or 53 in at least one eye and no CI-DME at baseline Post-hoc analysis of 3 step worsening/improvement on a Binocular DRSS Person-Level Scale, BCVA, and development of PDR 4 38 subjects excluded; NPDR Subset includes subjects at high risk
for progression to PDR
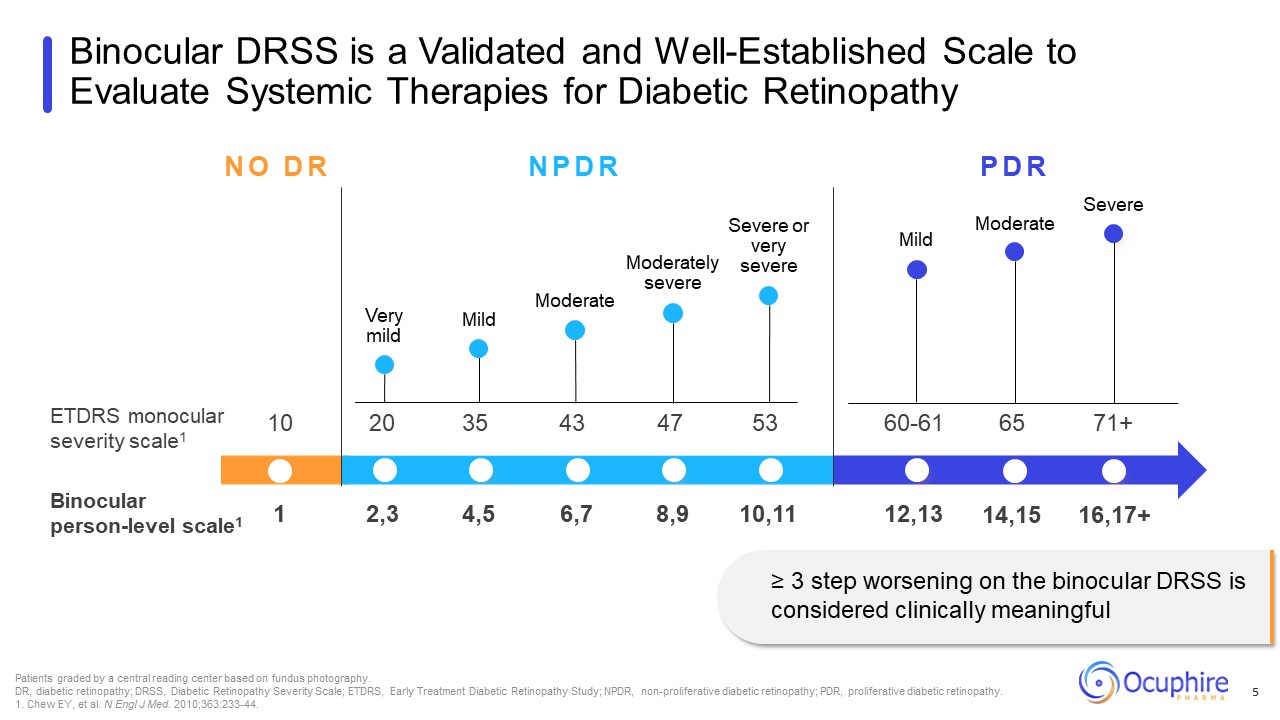
NO DR NPDR PDR ETDRS monocular severity scale1 Binocular person-level
scale1 Very mild Mild Moderate Moderately severe Severe or very severe Mild Patients graded by a central reading center based on fundus photography. DR, diabetic retinopathy; DRSS, Diabetic Retinopathy Severity Scale; ETDRS, Early
Treatment Diabetic Retinopathy Study; NPDR, non-proliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy. 1. Chew EY, et al. N Engl J Med. 2010;363:233-44. Binocular DRSS is a Validated and Well-Established Scale to
Evaluate Systemic Therapies for Diabetic Retinopathy 5 10 20 35 43 47 53 60-61 65 71+ Moderate Severe 2,3 4,5 6,7 8,9 10,11 12,13 14,15 16,17+ 1 ≥ 3 step worsening on the binocular DRSS is considered clinically
meaningful
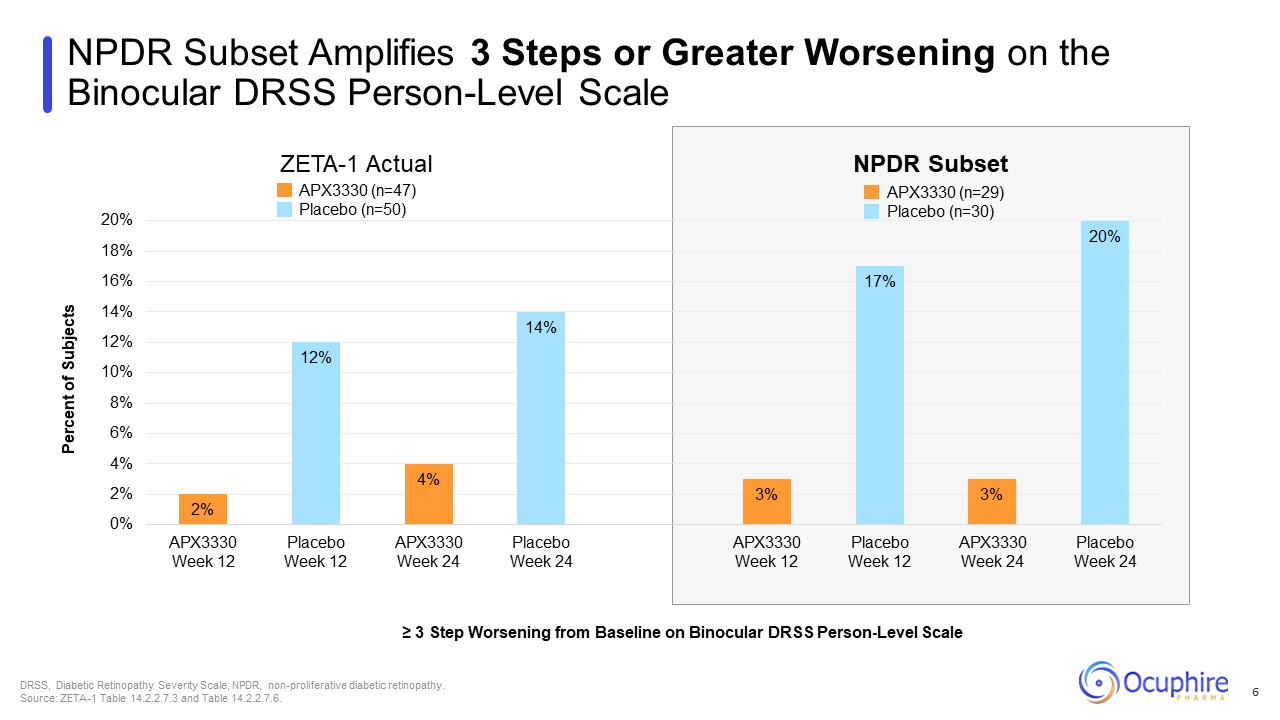
NPDR Subset Amplifies 3 Steps or Greater Worsening on the Binocular DRSS
Person-Level Scale ZETA-1 Actual NPDR Subset ≥ 3 Step Worsening from Baseline on Binocular DRSS Person-Level Scale DRSS, Diabetic Retinopathy Severity Scale; NPDR, non-proliferative diabetic retinopathy. Source: ZETA-1 Table 14.2.2.7.3
and Table 14.2.2.7.6. 6
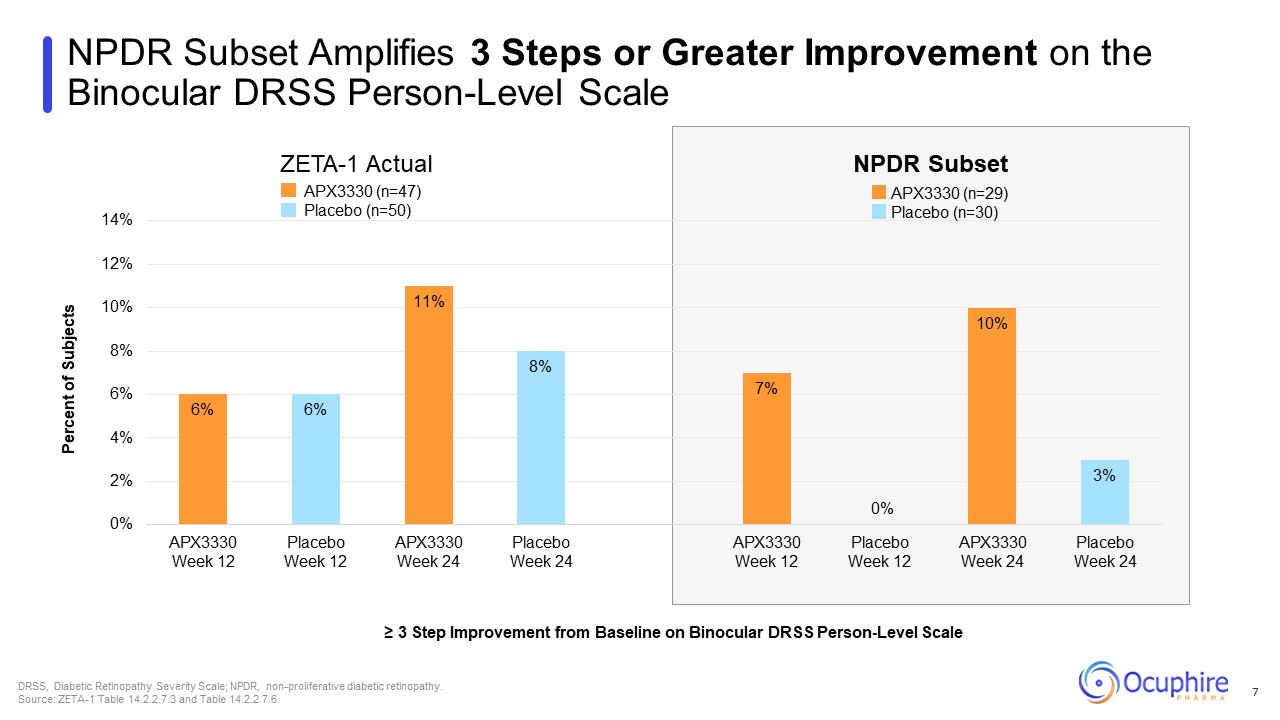
NPDR Subset Amplifies 3 Steps or Greater Improvement on the Binocular DRSS
Person-Level Scale ≥ 3 Step Improvement from Baseline on Binocular DRSS Person-Level Scale DRSS, Diabetic Retinopathy Severity Scale; NPDR, non-proliferative diabetic retinopathy. Source: ZETA-1 Table 14.2.2.7.3 and Table
14.2.2.7.6. ZETA-1 Actual NPDR Subset APX3330 (n=29) Placebo (n=30) APX3330 (n=47) Placebo (n=50) 7
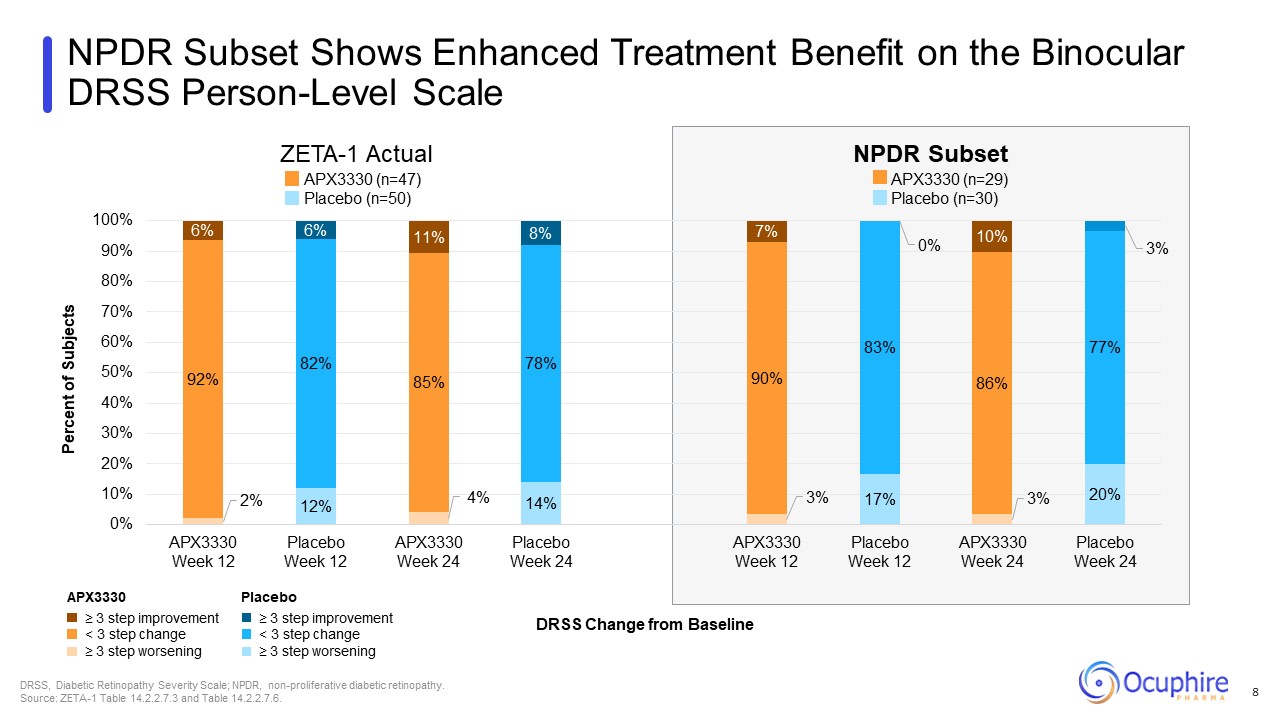
NPDR Subset Shows Enhanced Treatment Benefit on the Binocular DRSS Person-Level
Scale ≥ 3 step improvement < 3 step change ≥ 3 step worsening Placebo ≥ 3 step improvement < 3 step change ≥ 3 step worsening APX3330 DRSS Change from Baseline DRSS, Diabetic Retinopathy Severity Scale; NPDR,
non-proliferative diabetic retinopathy. Source: ZETA-1 Table 14.2.2.7.3 and Table 14.2.2.7.6. APX3330 (n=29) Placebo (n=30) APX3330 (n=47) Placebo (n=50) ZETA-1 Actual NPDR Subset 8
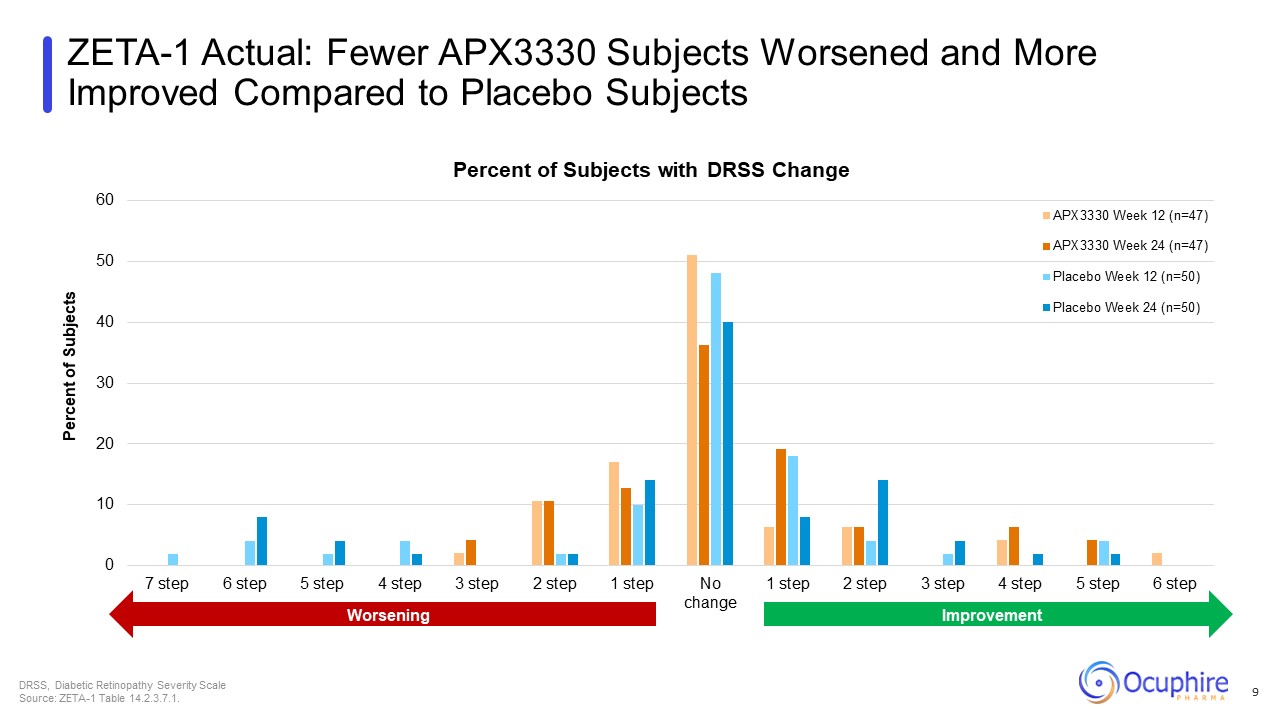
ZETA-1 Actual: Fewer APX3330 Subjects Worsened and More Improved Compared
to Placebo Subjects DRSS, Diabetic Retinopathy Severity Scale Source: ZETA-1 Table 14.2.3.7.1. 9 Worsening Improvement
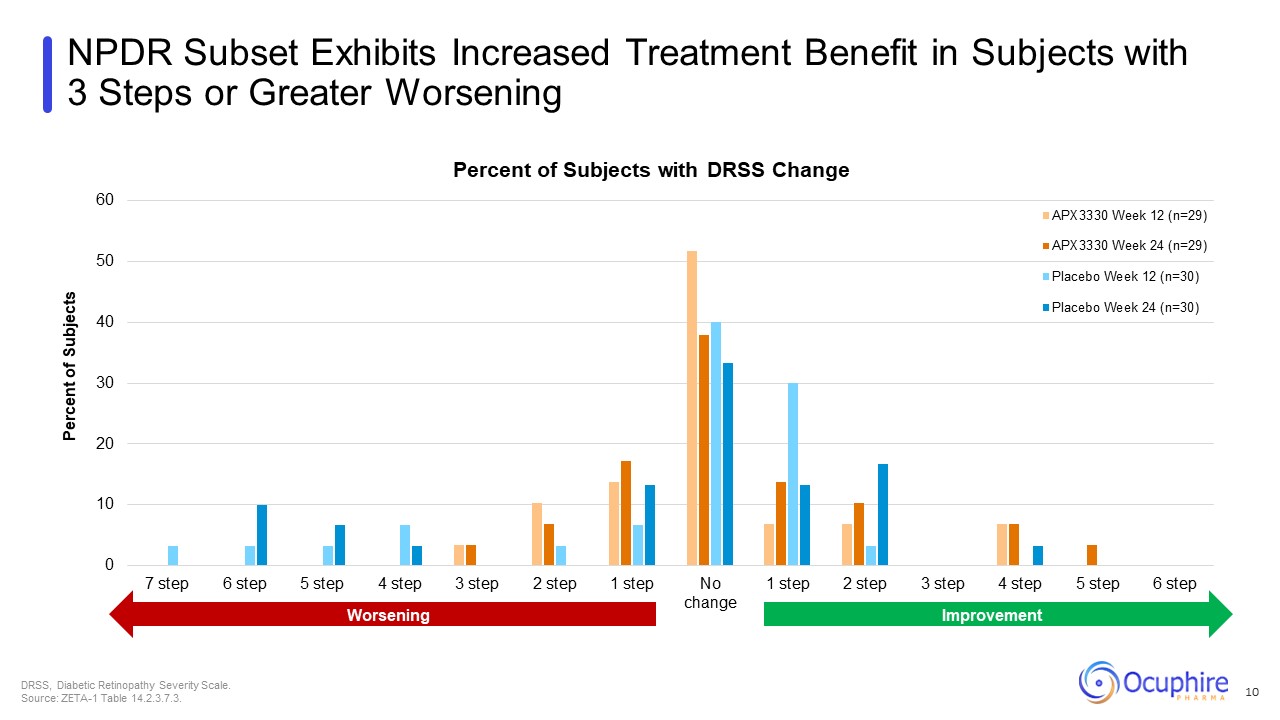
NPDR Subset Exhibits Increased Treatment Benefit in Subjects with 3 Steps or
Greater Worsening DRSS, Diabetic Retinopathy Severity Scale. Source: ZETA-1 Table 14.2.3.7.3. 10 Worsening Improvement
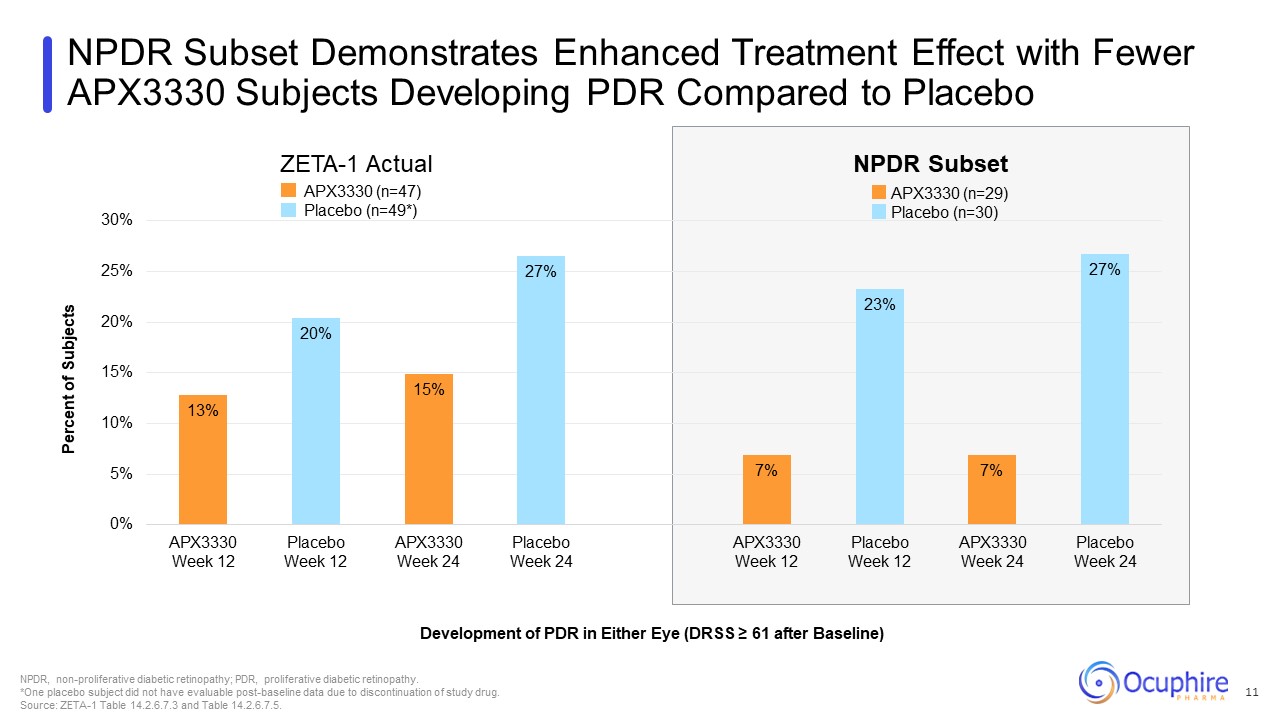
NPDR Subset Demonstrates Enhanced Treatment Effect with Fewer APX3330 Subjects
Developing PDR Compared to Placebo Development of PDR in Either Eye (DRSS ≥ 61 after Baseline) NPDR, non-proliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy. *One placebo subject did not have evaluable
post-baseline data due to discontinuation of study drug. Source: ZETA-1 Table 14.2.6.7.3 and Table 14.2.6.7.5. ZETA-1 Actual NPDR Subset APX3330 (n=29) Placebo (n=30) APX3330 (n=47) Placebo (n=49*) 11
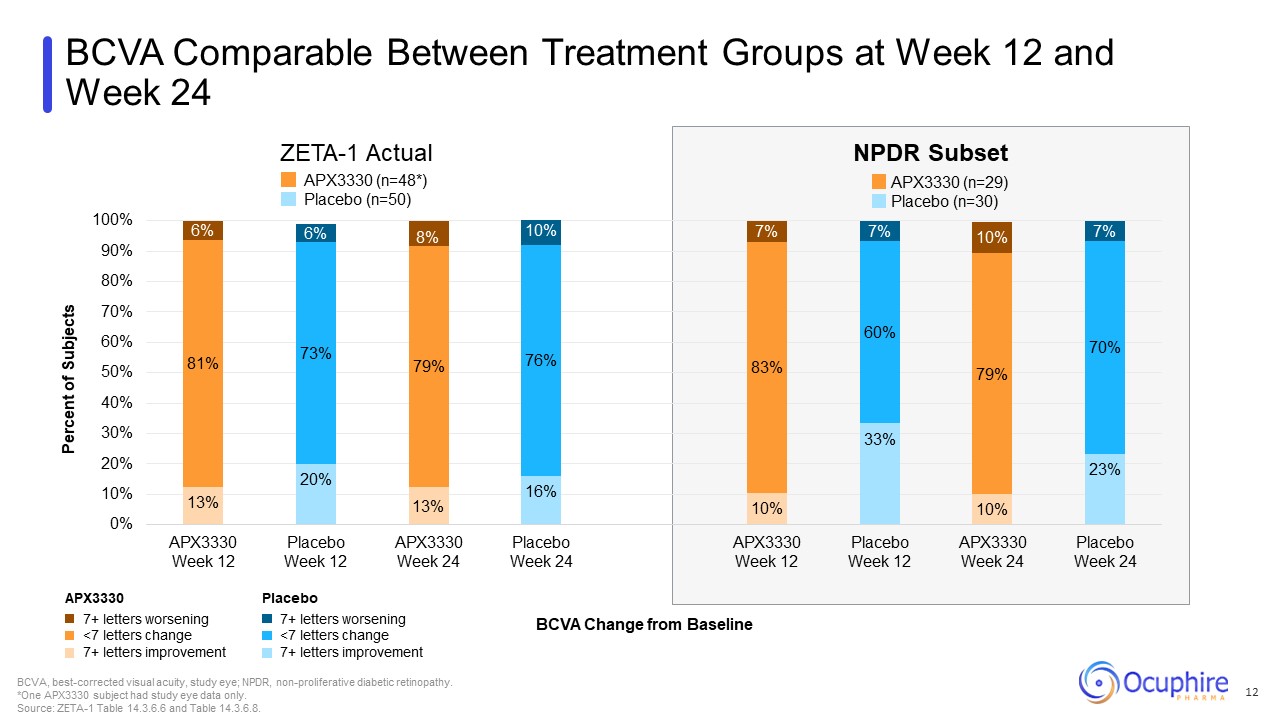
BCVA Comparable Between Treatment Groups at Week 12 and Week 24 7+ letters
worsening <7 letters change 7+ letters improvement Placebo 7+ letters worsening <7 letters change 7+ letters improvement APX3330 BCVA Change from Baseline BCVA, best-corrected visual acuity, study eye; NPDR, non-proliferative
diabetic retinopathy. *One APX3330 subject had study eye data only. Source: ZETA-1 Table 14.3.6.6 and Table 14.3.6.8. ZETA-1 Actual NPDR Subset APX3330 (n=29) Placebo (n=30) APX3330 (n=48*) Placebo (n=50) 12
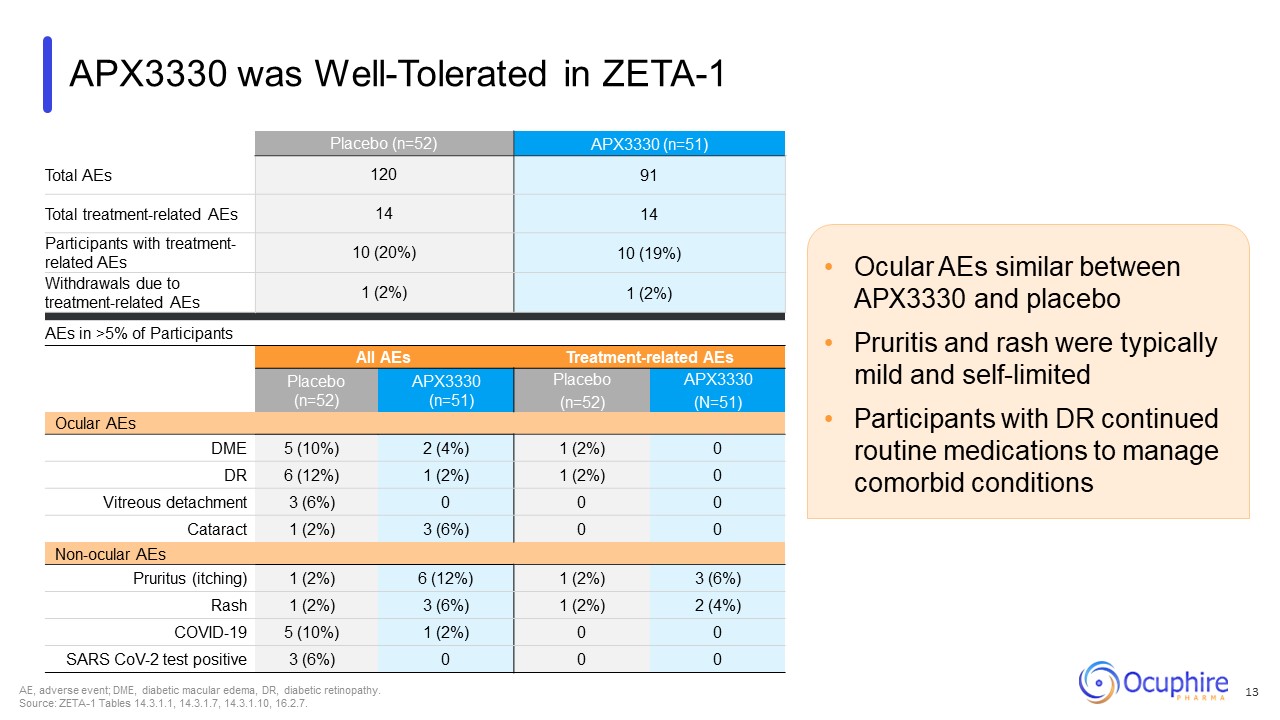
Ocular AEs similar between APX3330 and placebo Pruritis and rash were typically
mild and self-limited Participants with DR continued routine medications to manage comorbid conditions AE, adverse event; DME, diabetic macular edema, DR, diabetic retinopathy. Source: ZETA-1 Tables 14.3.1.1, 14.3.1.7, 14.3.1.10,
16.2.7. Placebo (n=52) APX3330 (n=51) Total AEs 120 91 Total treatment-related AEs 14 14 Participants with treatment-related AEs 10 (20%) 10 (19%) Withdrawals due to treatment-related AEs 1 (2%) 1 (2%) AEs in >5% of
Participants All AEs Treatment-related AEs Placebo (n=52) APX3330 (n=51) Placebo (n=52) APX3330 (N=51) Ocular AEs DME 5 (10%) 2 (4%) 1 (2%) 0 DR 6 (12%) 1 (2%) 1 (2%) 0 Vitreous detachment 3 (6%) 0 0 0 Cataract 1
(2%) 3 (6%) 0 0 Non-ocular AEs Pruritus (itching) 1 (2%) 6 (12%) 1 (2%) 3 (6%) Rash 1 (2%) 3 (6%) 1 (2%) 2 (4%) COVID-19 5 (10%) 1 (2%) 0 0 SARS CoV-2 test positive 3 (6%) 0 0 0 APX3330 was Well-Tolerated in
ZETA-1 13
NPDR Subset Demonstrates Potential Benefit of APX3330 in Patients with High Risk
NPDR NPDR subset suggests treatment effect on 3 steps or greater worsening on the binocular DRSS person-level scale 3% of APX3330 subjects compared to 20% of placebo subjects had 3 steps or greater worsening at Week 24 NPDR subset
suggests enhanced treatment effect on development of PDR 7% of APX3330 subjects compared to 27% of placebo subjects developed PDR (DRSS ≥ 61 after baseline in either eye) at Week 24 APX3330 was well-tolerated NPDR subset informs planned
clinical trial investigating APX3330 in slowing PDR conversion in high risk NPDR patients DRSS, Diabetic Retinopathy Severity Scale; NPDR, non-proliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy. 14