EXHIBIT 99.2
Published on October 22, 2024
Exhibit 99.2

Delivering on the Promise of Ophthalmic Gene Therapy for Rare Inherited Retinal
Diseases October 2024 Braydon, RDH12 patient

This presentation contains forward-looking statements within the meaning of the Private
Securities Litigation Reform Act of 1995. Such statements include, but are not limited to, statements concerning expectations regarding our cash runway, data from and future enrollment for our clinical trials, our pipeline of additional
indications, expectations of potential growth, and our expectations regarding the acquisition of Opus Genetics. These forward-looking statements relate to us, our business prospects and our results of operations and are subject to certain
risks and uncertainties posed by many factors and events that could cause our actual business, prospects and results of operations to differ materially from those anticipated by such forward-looking statements. Factors that could cause or
contribute to such differences include, but are not limited to, those described under the heading “Risk Factors” included in our Annual Report on Form 10-K and subsequent filings with the U.S. Securities and Exchange Commission (the “SEC”).
Readers are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date of this presentation. In some cases, you can identify forward-looking statements by the following words: “anticipate,”
“believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “ongoing,” “plan,” “potential,” “predict,” “project,” “should,” “will,” “would,” or the negative of these terms, or other comparable terminology, although not all
forward-looking statements contain these words. We undertake no obligation to revise any forward-looking statements in order to reflect events or circumstances that might subsequently arise. These forward-looking statements are based upon
Ocuphire’s current expectations and involve assumptions that may never materialize or may prove to be incorrect. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a
result of various risks and uncertainties, including, without limitation: the success and timing of regulatory submissions and pre-clinical and clinical trials, including enrollment and data readouts; regulatory requirements or developments;
changes to or unanticipated events in connection with clinical trial designs and regulatory pathways; delays or difficulties in the enrollment of patients in clinical trials; substantial competition and rapid technological change; our
development of sales and marketing infrastructure; future revenue losses and profitability; our relatively short operating history; changes in capital resource requirements; risks related to the inability of Ocuphire to obtain sufficient
additional capital to continue to advance its product candidates and its preclinical programs; domestic and worldwide legislative, regulatory, political and economic developments; employee misconduct; changes in market opportunities and
acceptance; reliance on third parties; future, potential product liability and securities litigation; system failures, unplanned events, or cyber incidents; the substantial number of shares subject to potential issuance associated with our
Equity Line of Credit arrangement; risks that our partnership with Viatris, or our other licensing arrangements, may not facilitate the commercialization or market acceptance of Ocuphire’s product candidates; future fluctuations in the market
price of our common stock; the success and timing of commercialization of any of Ocuphire’s product candidates; obtaining and maintaining Ocuphire’s intellectual property rights; and the success of Acquisitions and acquisitions. The
foregoing review of important factors that could cause actual events to differ from expectations should not be construed as exhaustive. Readers are urged to carefully review and consider the various disclosures made by us in this presentation
and in our reports filed with the SEC that advise interested parties of the risks and factors that may affect our business. All forward-looking statements contained in this presentation speak only as of the date on which they were
made. Ocuphire undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made. Disclosures and Forward-Looking Statements 2
Agenda – Acquisition of Opus Genetics Opening Remarks & Transaction
Overview George Magrath, MD Chief Executive Officer Opus Genetics Introduction Ben Yerxa, PhD President Pipeline Overview Ash Jayagopal, PhD Chief Scientific & Development Officer Q& A Opus Genetics
Management 1 2 3 4 3
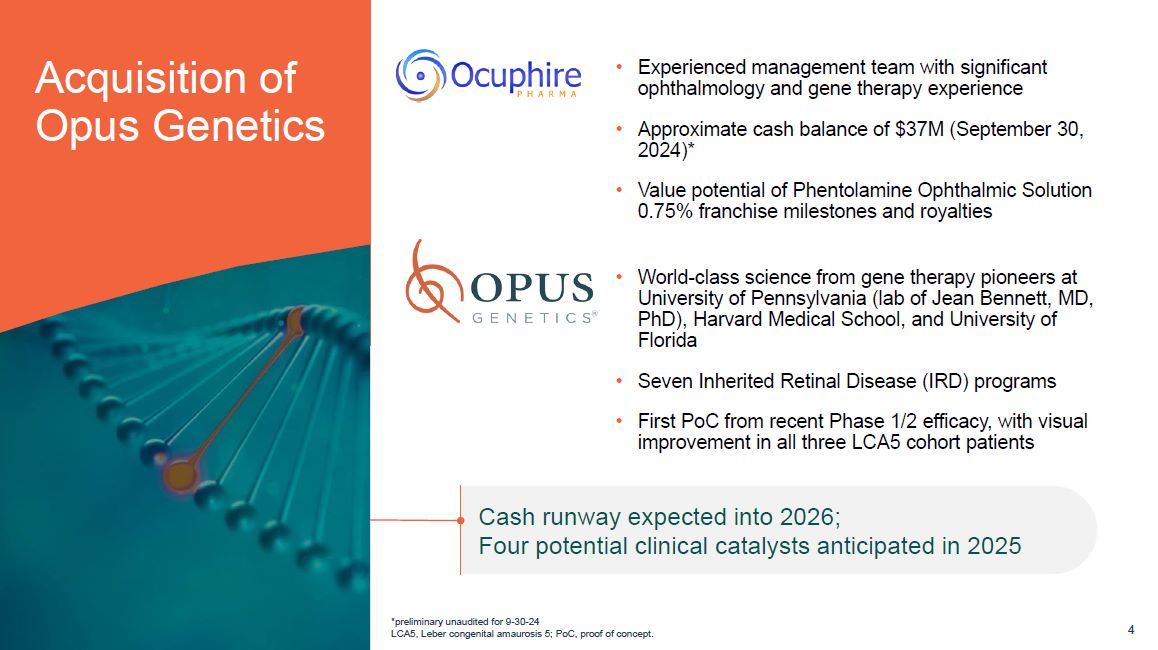
Acquisition of Opus Genetics Experienced management team with significant ophthalmology
and gene therapy experience Approximate cash balance of $37M (September 30, 2024)* Value potential of Phentolamine Ophthalmic Solution 0.75% franchise milestones and royalties World-class science from gene therapy pioneers at University
of Pennsylvania (lab of Jean Bennett, MD, PhD), Harvard Medical School, and University of Florida Seven Inherited Retinal Disease (IRD) programs First PoC from recent Phase 1/2 efficacy, with visual improvement in all three LCA5 cohort
patients Cash runway expected into 2026; Four potential clinical catalysts anticipated in 2025 *preliminary unaudited for 9-30-24 LCA5, Leber congenital amaurosis 5; PoC, proof of concept. 4
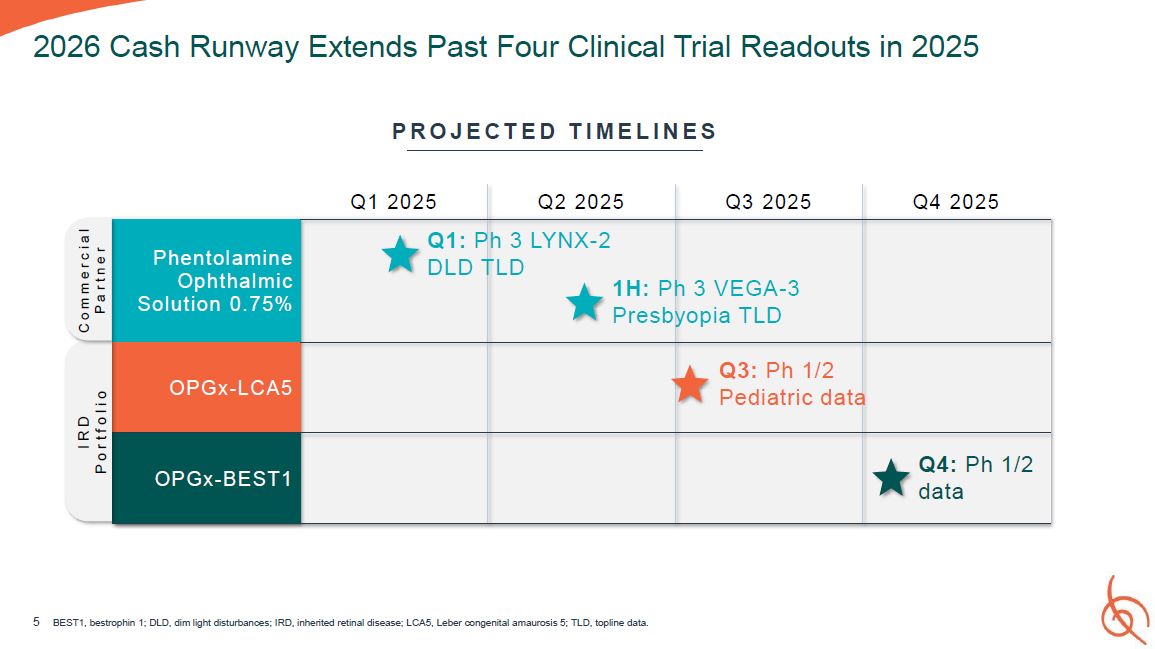
Q1 2025 Q2 2025 Q3 2025 Q4 2025 Phentolamine Ophthalmic Solution 0 . 75 % Q1:
P DLD T h 3 LYNX-2 LD 1H: P Presb h 3 VEGA-3 yopia TLD OPGx- LCA 5 Q3: Ph 1/2 Pediatric data OPGx- BEST1 Q4: Ph 1/2 data 5 BEST1, bestrophin 1; DLD, dim light disturbances; IRD, inherited retinal disease; LCA5, Leber congenital
amaurosis 5; TLD, topline data. 2026 Cash Runway Extends Past Four Clinical Trial Readouts in 2025 P R O J E C T E D T I M E L I N E S I R D P o r t f o l i o C o m m e r c i a l P a r t n e r

Ocuphire has completed acquisition of Opus in all-stock transaction Pro forma ownership
of 58% Ocuphire / 42% Opus Company will trade under new ticker of “IRD” Pro forma estimated cash and cash equivalents is $37M as of September 30, 2024, with an expected runway into 2026 Not including additional non-dilutive funding
expected from multiple sources Opus executive Ben Yerxa, PhD, joins the combined company as President and will also join the Board of Directors Jean Bennett, MD, PhD and Adrienne Graves, PhD will also join the Board of Directors of the
combined company, expanding to 9 directors Transaction Overview 6
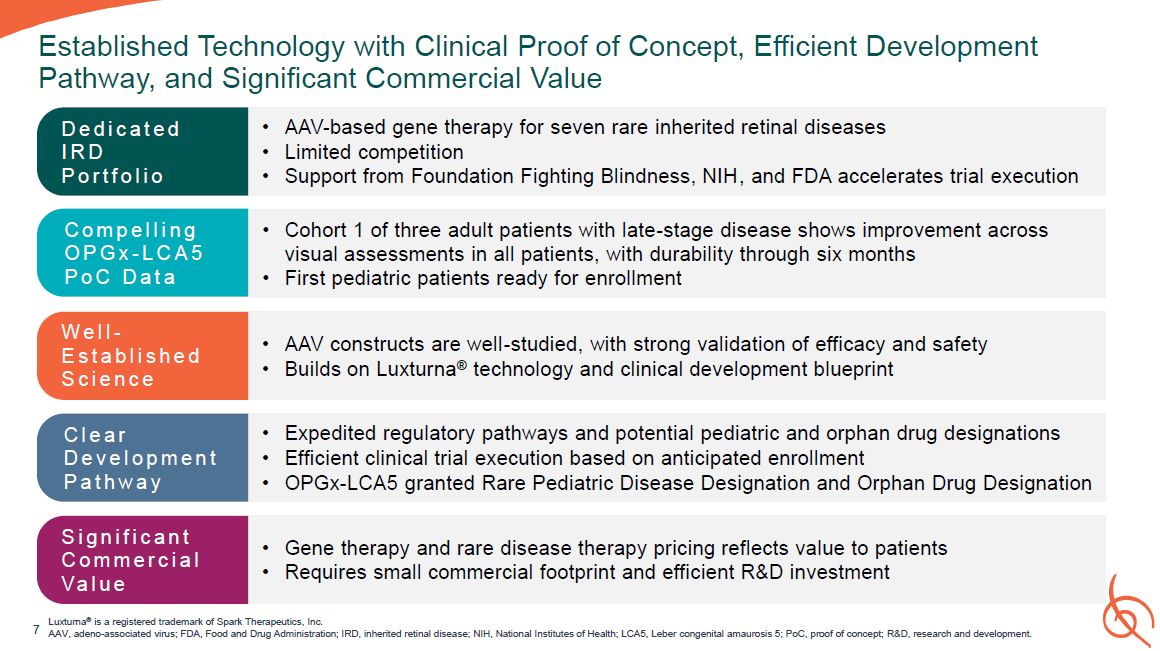
Established Technology with Clinical Proof of Concept, Efficient Development Pathway,
and Significant Commercial Value Luxturna® is a registered trademark of Spark Therapeutics, Inc. AAV, adeno-associated virus; FDA, Food and Drug Administration; IRD, inherited retinal disease; NIH, National Institutes of Health; LCA5, Leber
congenital amaurosis 5; PoC, proof of concept; R&D, research and development. AAV-based gene therapy for seven rare inherited retinal diseases Limited competition Support from Foundation Fighting Blindness, NIH, and FDA accelerates
trial execution D e d i c a t e d IRD P o r t f o l i o AAV constructs are well-studied, with strong validation of efficacy and safety Builds on Luxturna® technology and clinical development blueprint W e l l - E s t a b l i s h e d S
c i e n c e Expedited regulatory pathways and potential pediatric and orphan drug designations Efficient clinical trial execution based on anticipated enrollment OPGx-LCA5 granted Rare Pediatric Disease Designation and Orphan Drug
Designation C l e a r D e v e l o p m e n t P a t h w a y Gene therapy and rare disease therapy pricing reflects value to patients Requires small commercial footprint and efficient R&D investment S i g n i f i c a n t C o m m e r c i
a l Va l u e Cohort 1 of three adult patients with late-stage disease shows improvement across visual assessments in all patients, with durability through six months First pediatric patients ready for enrollment C o m p e l l i n g O P G
x - L C A 5 P o C D a t a 7

Fully Integrated Leadership Team with Decades of Expertise and Successful Track Record
of Development and Commercialization 8 George Magrath, MD, MBA, MS Chief Executive Officer Nirav Jhaveri, MBA Chief Financial Officer Joseph Schachle, MBA Chief Operating Officer Jean Bennett, MD, PhD Scientific Advisor Ben Yerxa,
PhD President Ash Jayagopal, PhD, MBA Chief Scientific & Development Officer
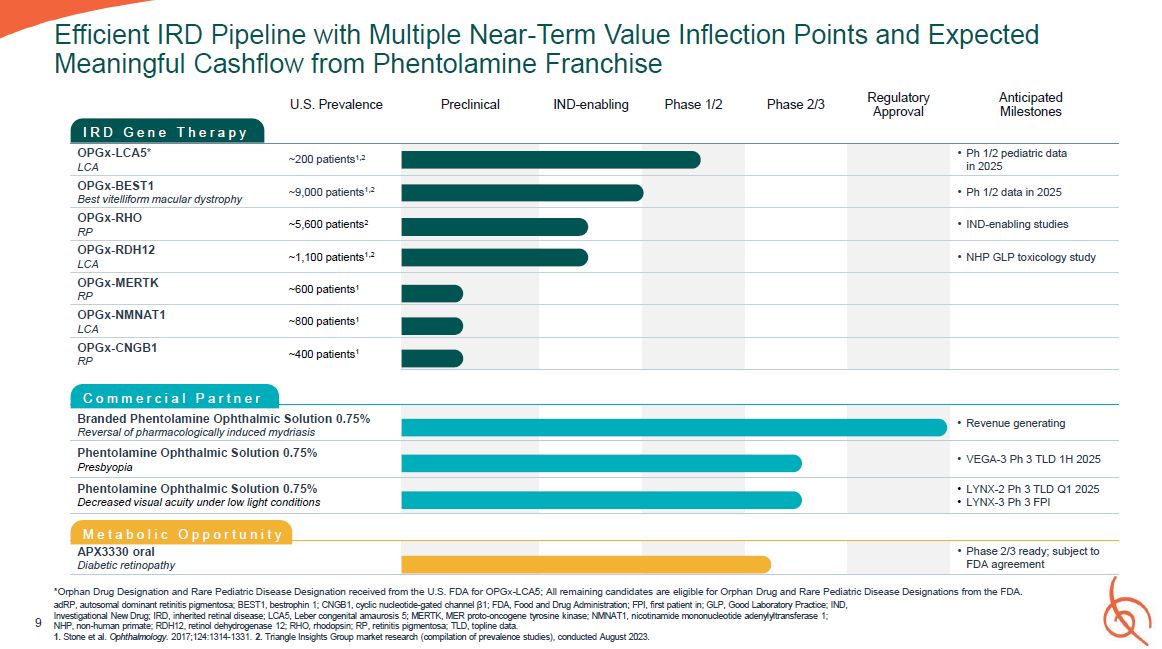
Efficient IRD Pipeline with Multiple Near-Term Value Inflection Points and Expected
Meaningful Cashflow from Phentolamine Franchise *Orphan Drug Designation and Rare Pediatric Disease Designation received from the U.S. FDA for OPGx-LCA5; All remaining candidates are eligible for Orphan Drug and Rare Pediatric Disease
Designations from the FDA. adRP, autosomal dominant retinitis pigmentosa; BEST1, bestrophin 1; CNGB1, cyclic nucleotide-gated channel β1; FDA, Food and Drug Administration; FPI, first patient in; GLP, Good Laboratory Practice; IND,
Investigational New Drug; IRD, inherited retinal disease; LCA5, Leber congenital amaurosis 5; MERTK, MER proto-oncogene tyrosine kinase; NMNAT1, nicotinamide mononucleotide adenylyltransferase 1; NHP, non-human primate; RDH12, retinol
dehydrogenase 12; RHO, rhodopsin; RP, retinitis pigmentosa; TLD, topline data. 1. Stone et al. Ophthalmology. 2017;124:1314-1331. 2. Triangle Insights Group market research (compilation of prevalence studies), conducted August 2023. 9 I R
D G e n e T h e r a p y U.S. Prevalence Preclinical IND-enabling Phase 1/2 Phase 2/3 Regulatory Approval Anticipated Milestones OPGx-LCA5* LCA ~200 patients1,2 Ph 1/2 pediatric data in 2025 OPGx-BEST1 Best vitelliform macular
dystrophy ~9,000 patients1,2 Ph 1/2 data in 2025 OPGx-RHO RP ~5,600 patients2 IND-enabling studies OPGx-RDH12 LCA ~1,100 patients1,2 NHP GLP toxicology study OPGx-MERTK RP ~600 patients1 OPGx-NMNAT1 LCA ~800
patients1 OPGx-CNGB1 RP ~400 patients1 C o m m e r c i a l P a r t n e r Branded Phentolamine Ophthalmic Solution 0.75% Reversal of pharmacologically induced mydriasis Revenue generating Phentolamine Ophthalmic Solution
0.75% Presbyopia VEGA-3 Ph 3 TLD 1H 2025 Phentolamine Ophthalmic Solution 0.75% Decreased visual acuity under low light conditions LYNX-2 Ph 3 TLD Q1 2025 LYNX-3 Ph 3 FPI M e t a b o l i c O p p o r t u n i t y APX3330 oral Diabetic
retinopathy Phase 2/3 ready; subject to FDA agreement

OPGx-LCA5 Phase 1/2 Gene Therapy for LCA5 Alan, LCA5 patient LCA5, Leber congenital
amaurosis 5.
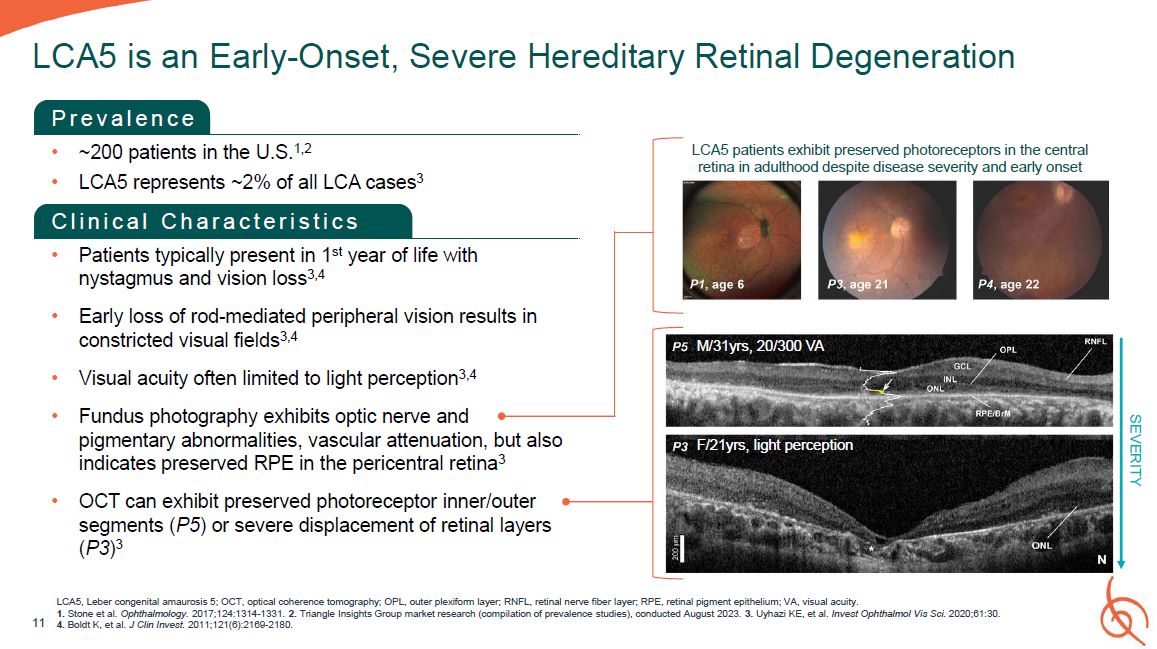
P r e v a l e n c e ~200 patients in the U.S.1,2 LCA5 represents ~2% of all LCA
cases3 C l i n i c a l C h a r a c t e r i s t i c s Patients typically present in 1st year of life with nystagmus and vision loss3,4 Early loss of rod-mediated peripheral vision results in constricted visual fields3,4 Visual acuity often
limited to light perception3,4 Fundus photography exhibits optic nerve and pigmentary abnormalities, vascular attenuation, but also indicates preserved RPE in the pericentral retina3 OCT can exhibit preserved photoreceptor inner/outer
segments (P5) or severe displacement of retinal layers (P3)3 11 LCA5 is an Early-Onset, Severe Hereditary Retinal Degeneration SEVERITY M/31yrs, 20/300 VA F/21yrs, light perception LCA5 patients exhibit preserved photoreceptors in the
central retina in adulthood despite disease severity and early onset LCA5, Leber congenital amaurosis 5; OCT, optical coherence tomography; OPL, outer plexiform layer; RNFL, retinal nerve fiber layer; RPE, retinal pigment epithelium; VA,
visual acuity. 1. Stone et al. Ophthalmology. 2017;124:1314-1331. 2. Triangle Insights Group market research (compilation of prevalence studies), conducted August 2023. 3. Uyhazi KE, et al. Invest Ophthalmol Vis Sci. 2020;61:30. 4. Boldt K,
et al. J Clin Invest. 2011;121(6):2169-2180.
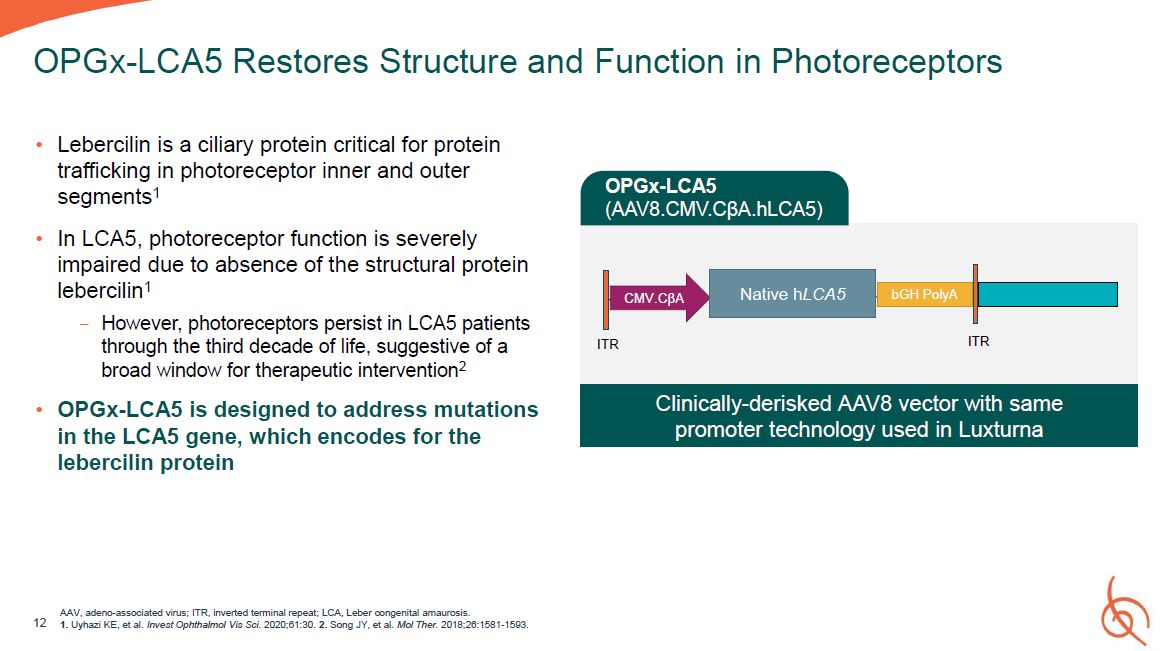
Lebercilin is a ciliary protein critical for protein trafficking in photoreceptor inner
and outer segments1 In LCA5, photoreceptor function is severely impaired due to absence of the structural protein lebercilin1 − However, photoreceptors persist in LCA5 patients through the third decade of life, suggestive of a broad window
for therapeutic intervention2 OPGx-LCA5 is designed to address mutations in the LCA5 gene, which encodes for the lebercilin protein AAV, adeno-associated virus; ITR, inverted terminal repeat; LCA, Leber congenital amaurosis. 12 1. Uyhazi
KE, et al. Invest Ophthalmol Vis Sci. 2020;61:30. 2. Song JY, et al. Mol Ther. 2018;26:1581-1593. OPGx-LCA5 Restores Structure and Function in Photoreceptors CMV.CβA Native hLCA5 bGH
PolyA ITR ITR OPGx-LCA5 (AAV8.CMV.CβA.hLCA5) Clinically-derisked AAV8 vector with same promoter technology used in Luxturna
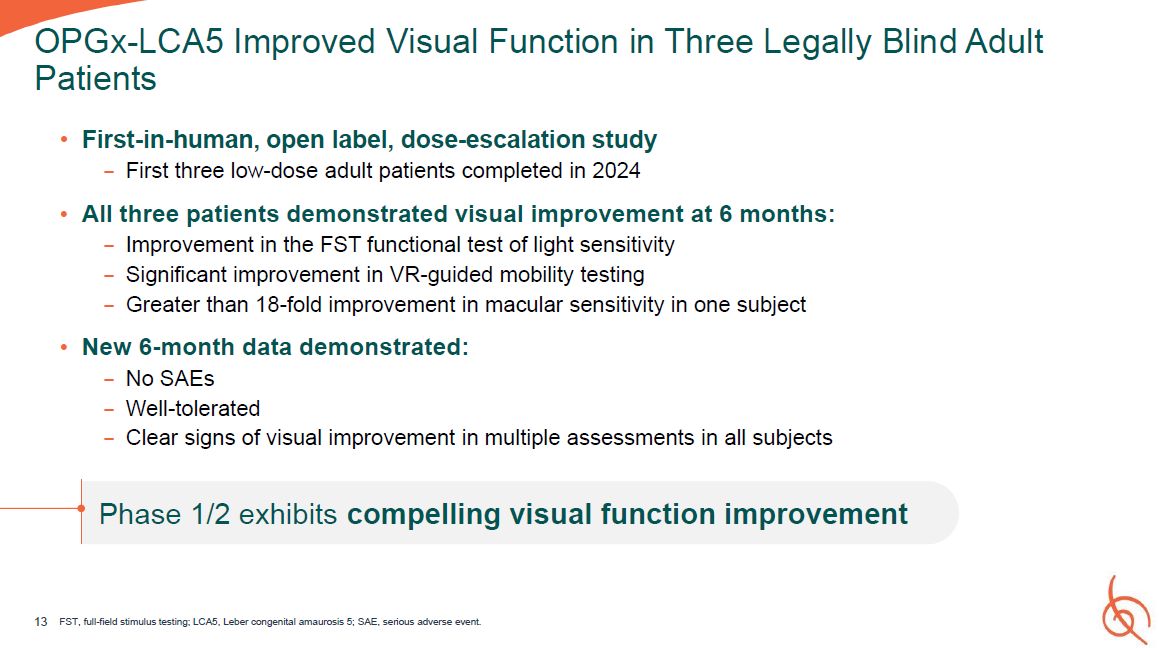
First-in-human, open label, dose-escalation study − First three low-dose adult patients
completed in 2024 All three patients demonstrated visual improvement at 6 months: − Improvement in the FST functional test of light sensitivity − Significant improvement in VR-guided mobility testing − Greater than 18-fold improvement in
macular sensitivity in one subject New 6-month data demonstrated: − No SAEs − Well-tolerated − Clear signs of visual improvement in multiple assessments in all subjects Phase 1/2 exhibits compelling visual function improvement 13 FST,
full-field stimulus testing; LCA5, Leber congenital amaurosis 5; SAE, serious adverse event. OPGx-LCA5 Improved Visual Function in Three Legally Blind Adult Patients
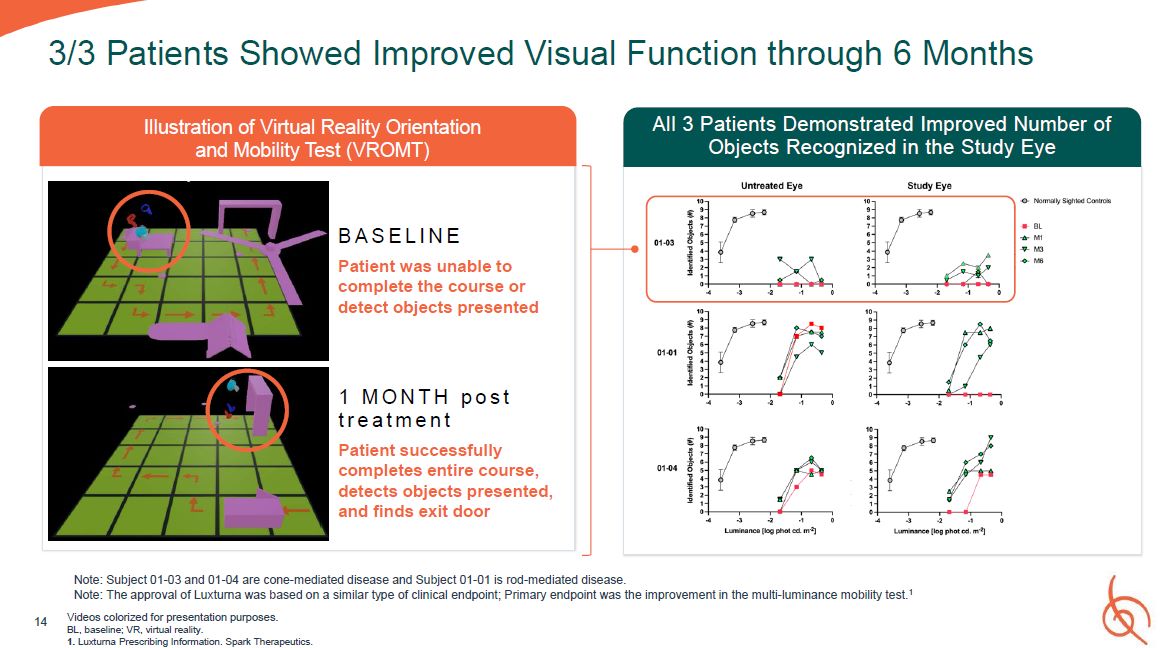
3/3 Patients Showed Improved Visual Function through 6 Months Videos colorized for
presentation purposes. BL, baseline; VR, virtual reality. 1. Luxturna Prescribing Information. Spark Therapeutics. Note: Subject 01-03 and 01-04 are cone-mediated disease and Subject 01-01 is rod-mediated disease. Note: The approval of
Luxturna was based on a similar type of clinical endpoint; Primary endpoint was the improvement in the multi-luminance mobility test.1 All 3 Patients Demonstrated Improved Number of Objects Recognized in the Study Eye 14 B A S E L I N
E Patient was unable to complete the course or detect objects presented 1 M O N T H p o s t t r e a t m e n t Patient successfully completes entire course, detects objects presented, and finds exit door Illustration of Virtual Reality
Orientation and Mobility Test (VROMT)
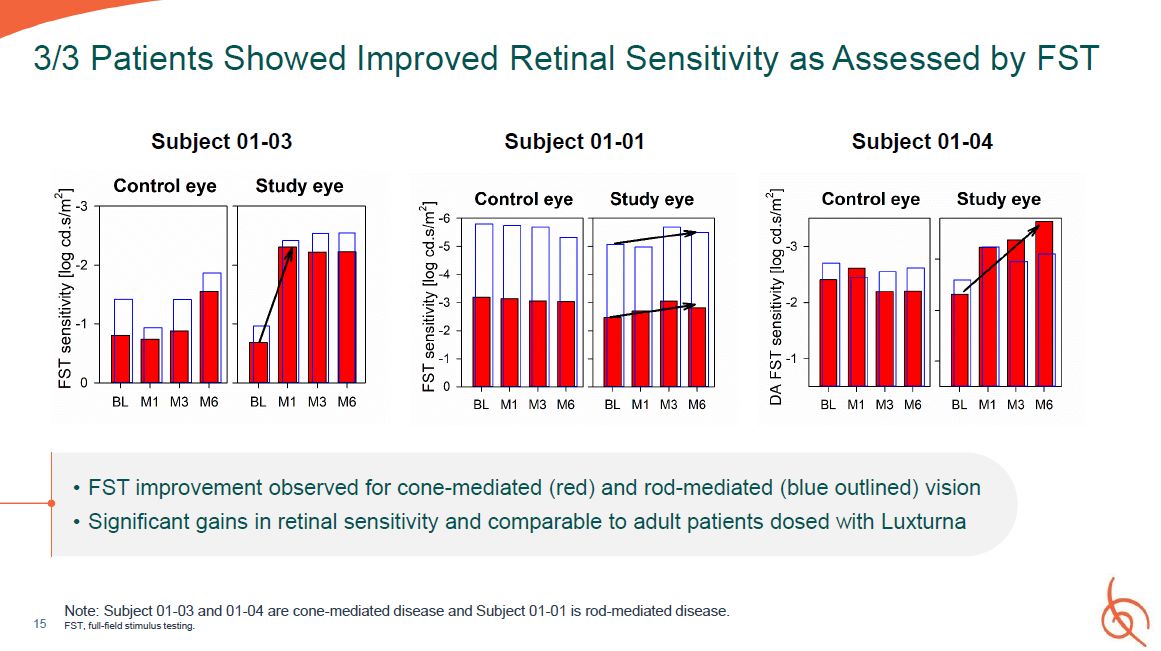
15 3/3 Patients Showed Improved Retinal Sensitivity as Assessed by FST Subject
01-03 Subject 01-01 Subject 01-04 FST improvement observed for cone-mediated (red) and rod-mediated (blue outlined) vision Significant gains in retinal sensitivity and comparable to adult patients dosed with Luxturna Note: Subject 01-03
and 01-04 are cone-mediated disease and Subject 01-01 is rod-mediated disease. FST, full-field stimulus testing.

Ready for enrollment of pediatric patients in Q1 2025, with preliminary data expected
in Q3 2025 FDA Office of Orphan Drug Products grant awarded to support Phase 1/2 trial Rare Pediatric Disease Designation and Orphan Drug Designation received from the FDA, which confers eligibility for Priority Review Voucher upon BLA
approval Accelerated clinical development pathway to approval may be appropriate if similar efficacy is demonstrated in pediatric patients 16 OPGx-LCA5 Phase 1/2 Next Steps: Ready to Enroll Pediatric Subjects BLA, Biologics License
Application; FDA, Food and Drug Administration; LCA5, Leber congenital amaurosis 5.

OPGx-BEST1 Phase 1/2-Ready Gene Therapy for BEST1-associated Disease BEST1, bestrophin
1.
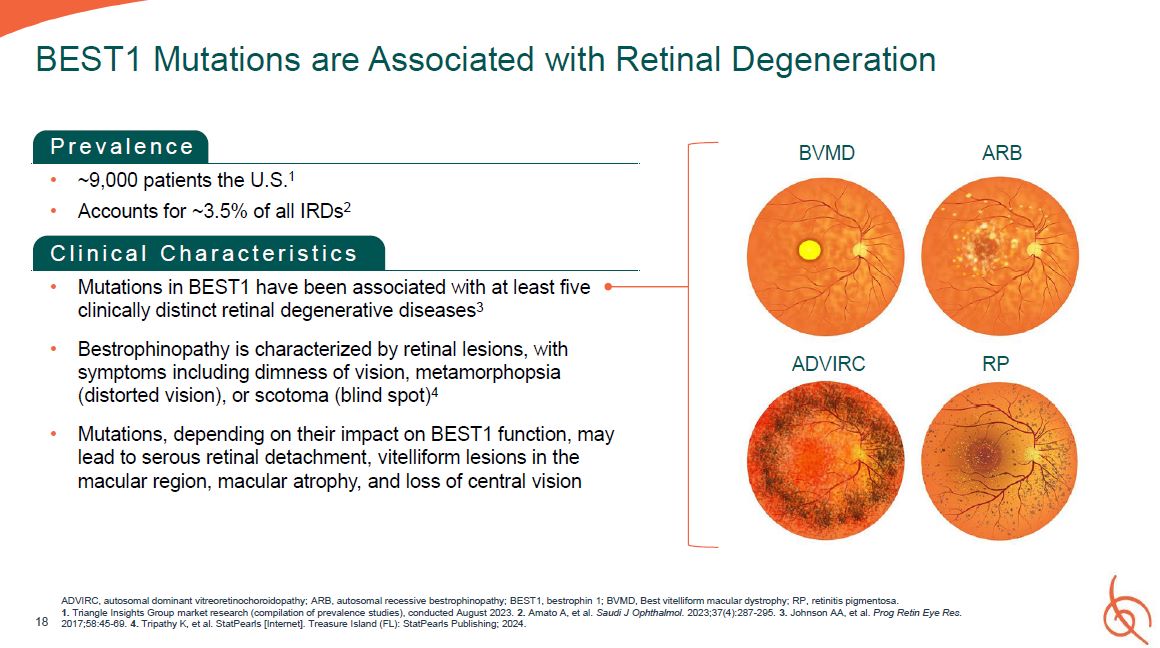
P r e v a l e n c e ~9,000 patients the U.S.1 Accounts for ~3.5% of all IRDs2 C l i n
i c a l C h a r a c t e r i s t i c s Mutations in BEST1 have been associated with at least five clinically distinct retinal degenerative diseases3 Bestrophinopathy is characterized by retinal lesions, with symptoms including dimness of
vision, metamorphopsia (distorted vision), or scotoma (blind spot)4 Mutations, depending on their impact on BEST1 function, may lead to serous retinal detachment, vitelliform lesions in the macular region, macular atrophy, and loss of
central vision 18 BEST1 Mutations are Associated with Retinal Degeneration BVMD ARB ADVIRC RP ADVIRC, autosomal dominant vitreoretinochoroidopathy; ARB, autosomal recessive bestrophinopathy; BEST1, bestrophin 1; BVMD, Best vitelliform
macular dystrophy; RP, retinitis pigmentosa. 1. Triangle Insights Group market research (compilation of prevalence studies), conducted August 2023. 2. Amato A, et al. Saudi J Ophthalmol. 2023;37(4):287-295. 3. Johnson AA, et al. Prog Retin
Eye Res. 2017;58:45-69. 4. Tripathy K, et al. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024.
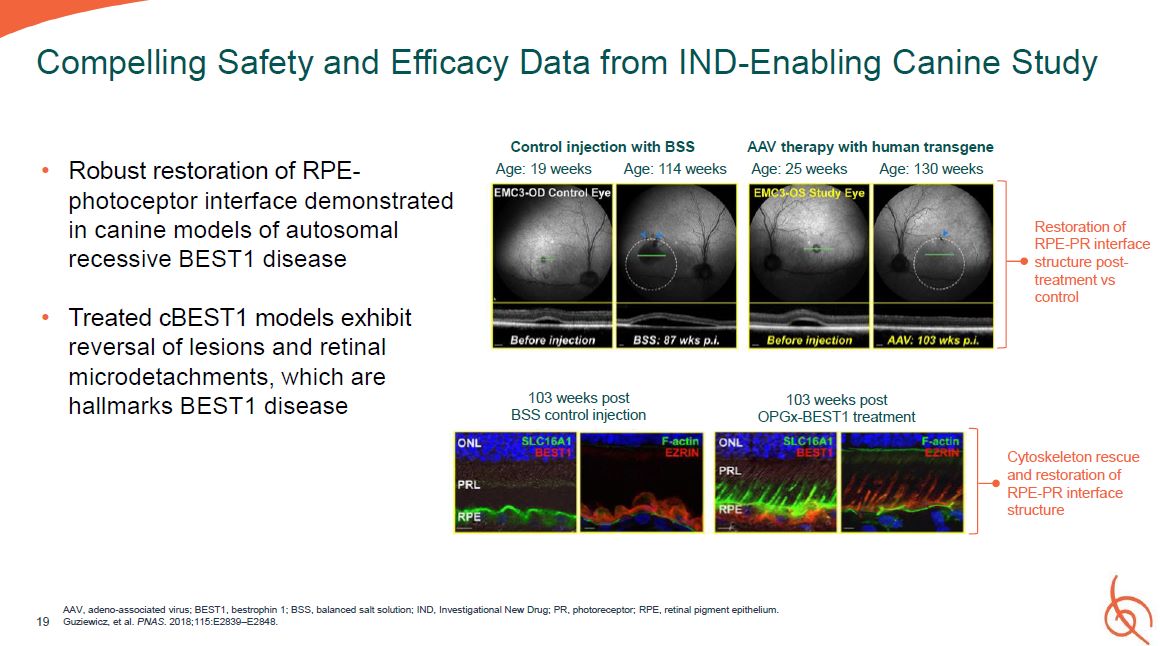
19 Compelling Safety and Efficacy Data from IND-Enabling Canine Study Robust
restoration of RPE- photoceptor interface demonstrated in canine models of autosomal recessive BEST1 disease Treated cBEST1 models exhibit reversal of lesions and retinal microdetachments, which are hallmarks BEST1 disease 103 weeks
post BSS control injection 103 weeks post OPGx-BEST1 treatment Cytoskeleton rescue and restoration of RPE-PR interface structure Control injection with BSS Age: 19 weeks Age: 114 weeks AAV therapy with human transgene Age: 25 weeks
Age: 130 weeks Restoration of RPE-PR interface structure post- treatment vs control AAV, adeno-associated virus; BEST1, bestrophin 1; BSS, balanced salt solution; IND, Investigational New Drug; PR, photoreceptor; RPE, retinal pigment
epithelium. Guziewicz, et al. PNAS. 2018;115:E2839–E2848.

20 OPGx-BEST1 well-tolerated in toxicology studies; human dose and NOAEL
established BEST1 AAV GMP drug product ready for human dosing; subject to regulatory clearance Positive feedback from EU regulators for Phase 1/2 clinical trial design in Germany Ready to initiate Phase 1/2 clinical trial in Germany, with
data anticipated in Q4 2025; subject to regulatory clearance AAV, adeno-associated virus; BEST1, bestrophin 1; EU, European Union; GMP, good manufacturing practices; NOAEL, no-observed-adverse-effect-level. Data from OPGx-BEST1 Phase 1/2
Study Expected in Q4 2025
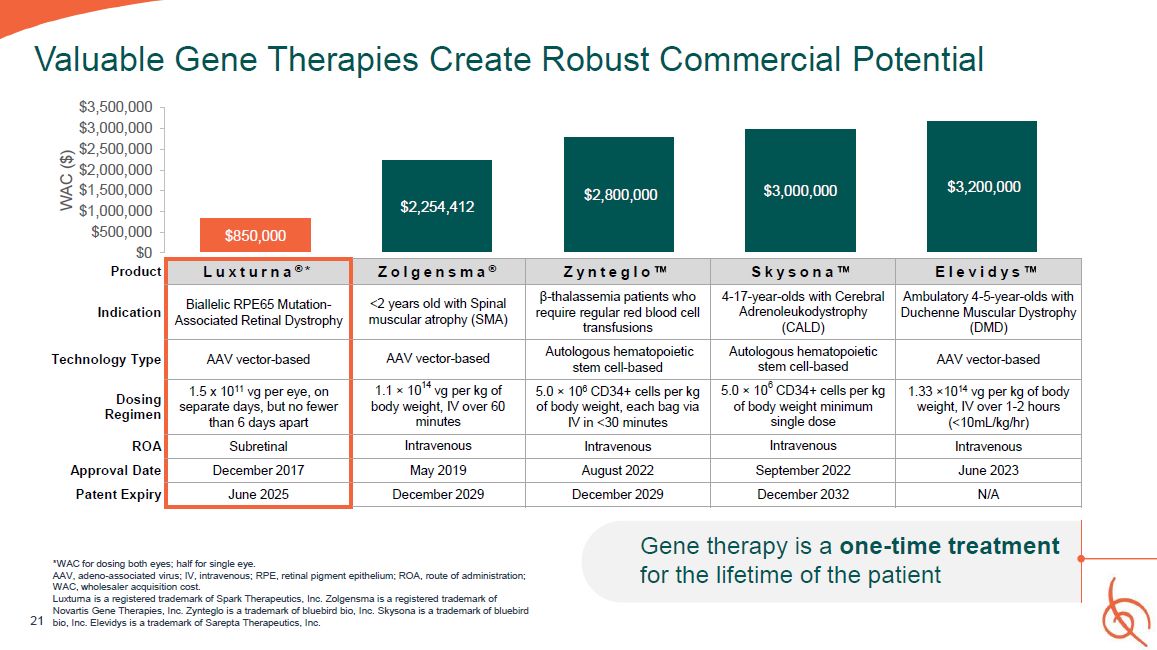
21 Valuable Gene Therapies Create Robust Commercial
Potential $850,000 $2,254,412 $2,800,000 $3,000,000 $3,200,000 $3,000,000 $2,500,000 $2,000,000 $1,500,000 $1,000,000 $500,000 $0 $3,500,000 WAC ($) Product L u x t u r n a ® * Z o l g e n s m a ® Z y n t e g l o S k y s o
n a E l e v i d y s Indication Biallelic RPE65 Mutation- Associated Retinal Dystrophy <2 years old with Spinal muscular atrophy (SMA) β-thalassemia patients who require regular red blood cell transfusions 4-17-year-olds with
Cerebral Adrenoleukodystrophy (CALD) Ambulatory 4-5-year-olds with Duchenne Muscular Dystrophy (DMD) Technology Type AAV vector-based AAV vector-based Autologous hematopoietic stem cell-based Autologous hematopoietic stem
cell-based AAV vector-based Dosing Regimen 1.5 x 1011 vg per eye, on separate days, but no fewer than 6 days apart 1.1 × 1014 vg per kg of body weight, IV over 60 minutes 5.0 × 106 CD34+ cells per kg of body weight, each bag via IV in
<30 minutes 5.0 × 106 CD34+ cells per kg of body weight minimum single dose 1.33 ×1014 vg per kg of body weight, IV over 1-2 hours (<10mL/kg/hr) ROA Subretinal Intravenous Intravenous Intravenous Intravenous Approval
Date December 2017 May 2019 August 2022 September 2022 June 2023 Patent Expiry June 2025 December 2029 December 2029 December 2032 N/A Gene therapy is a one-time treatment for the lifetime of the patient *WAC for dosing both
eyes; half for single eye. AAV, adeno-associated virus; IV, intravenous; RPE, retinal pigment epithelium; ROA, route of administration; WAC, wholesaler acquisition cost. Luxturna is a registered trademark of Spark Therapeutics, Inc.
Zolgensma is a registered trademark of Novartis Gene Therapies, Inc. Zynteglo is a trademark of bluebird bio, Inc. Skysona is a trademark of bluebird bio, Inc. Elevidys is a trademark of Sarepta Therapeutics, Inc.

Candidates that Fuel Our Future Growth Phentolamine Ophthalmic Solution
0.75% Franchise and APX3330
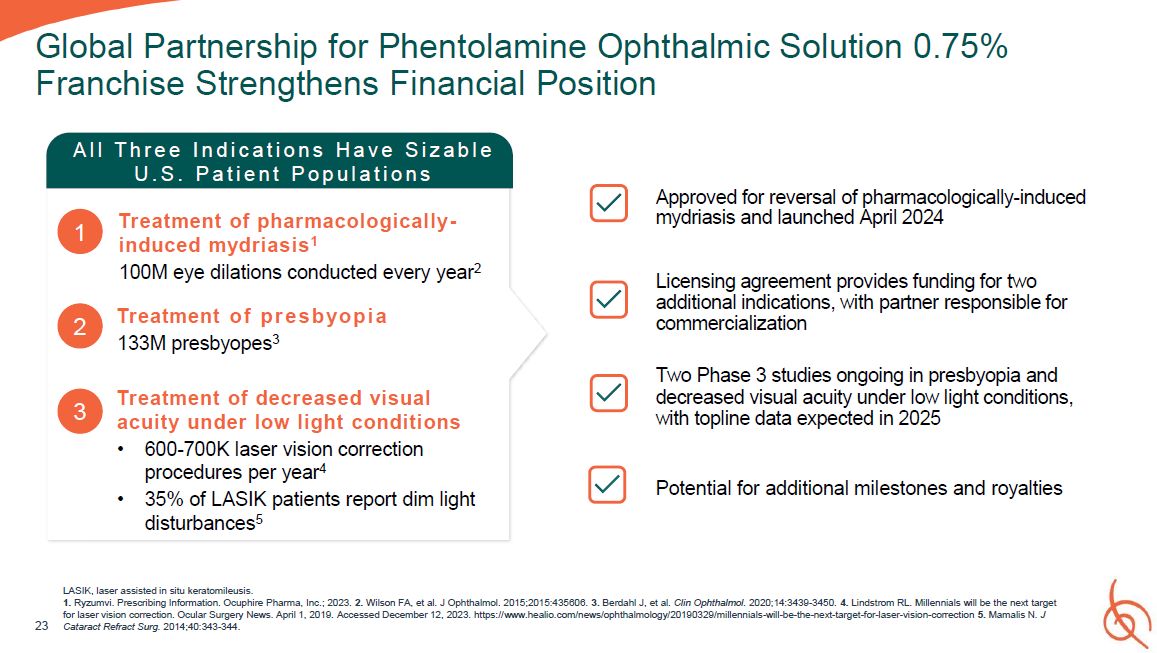
LASIK, laser assisted in situ keratomileusis. 1. Ryzumvi. Prescribing Information.
Ocuphire Pharma, Inc.; 2023. 2. Wilson FA, et al. J Ophthalmol. 2015;2015:435606. 3. Berdahl J, et al. Clin Ophthalmol. 2020;14:3439-3450. 4. Lindstrom RL. Millennials will be the next target for laser vision correction. Ocular Surgery News.
April 1, 2019. Accessed December 12, 2023. https://www.healio.com/news/ophthalmology/20190329/millennials-will-be-the-next-target-for-laser-vision-correction 5. Mamalis N. J 23 Cataract Refract Surg. 2014;40:343-344. Global Partnership for
Phentolamine Ophthalmic Solution 0.75% Franchise Strengthens Financial Position Approved for reversal of pharmacologically-induced mydriasis and launched April 2024 Licensing agreement provides funding for two additional indications, with
partner responsible for commercialization Two Phase 3 studies ongoing in presbyopia and decreased visual acuity under low light conditions, with topline data expected in 2025 Potential for additional milestones and royalties A l l T h r e
e I n d i c a t i o n s H a v e S i z a b l e U . S . P a t i e n t P o p u l a t i o n s 1 Treatment of pharmacologically- induced mydriasis1 100M eye dilations conducted every year2 2 3 Treatment o f p r e s b yo p i a 133M
presbyopes3 Treatment of decreased visual acuity under low light conditions 600-700K laser vision correction procedures per year4 35% of LASIK patients report dim light disturbances5
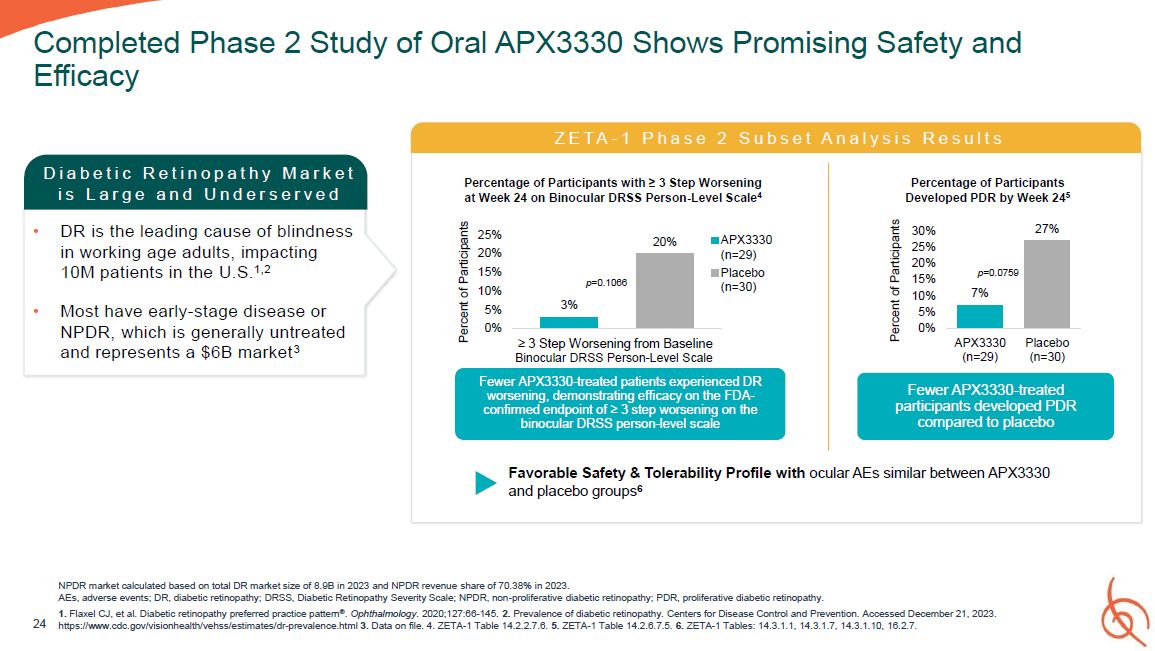
24 Completed Phase 2 Study of Oral APX3330 Shows Promising Safety and Efficacy D i a b
e t i c R e t i n o p a t h y M a r k e t i s L a r g e a n d U n d e r s e r v e d DR is the leading cause of blindness in working age adults, impacting 10M patients in the U.S.1,2 Most have early-stage disease or NPDR, which is generally
untreated and represents a $6B market3 NPDR market calculated based on total DR market size of 8.9B in 2023 and NPDR revenue share of 70.38% in 2023. AEs, adverse events; DR, diabetic retinopathy; DRSS, Diabetic Retinopathy Severity Scale;
NPDR, non-proliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy. 1. Flaxel CJ, et al. Diabetic retinopathy preferred practice pattern®. Ophthalmology. 2020;127:66-145. 2. Prevalence of diabetic retinopathy. Centers for
Disease Control and Prevention. Accessed December 21, 2023. https://www.cdc.gov/visionhealth/vehss/estimates/dr-prevalence.html 3. Data on file. 4. ZETA-1 Table 14.2.2.7.6. 5. ZETA-1 Table 14.2.6.7.5. 6. ZETA-1 Tables: 14.3.1.1, 14.3.1.7,
14.3.1.10, 16.2.7. Z E T A - 1 P h a s e 2 S u b s e t A n a l y s i s R e s u l t s Favorable Safety & Tolerability Profile with ocular AEs similar between APX3330 and placebo groups6 3% 20% 25% 20% 15% 10% 5% 0% Percent of
Participants ≥ 3 Step Worsening from Baseline Binocular DRSS Person-Level Scale Fewer APX3330-treated patients experienced DR worsening, demonstrating efficacy on the FDA- confirmed endpoint of ≥ 3 step worsening on the binocular DRSS
person-level scale APX3330 (n=29) Placebo (n=30) Percentage of Participants with ≥ 3 Step Worsening at Week 24 on Binocular DRSS Person-Level Scale4 p=0.1066 27% 30% 25% 20% 15% 10% 5% 0% APX3330 (n=29) Placebo (n=30) Percent
of Participants Fewer APX3330-treated participants developed PDR compared to placebo Percentage of Participants Developed PDR by Week 245 p=0.0759 7%
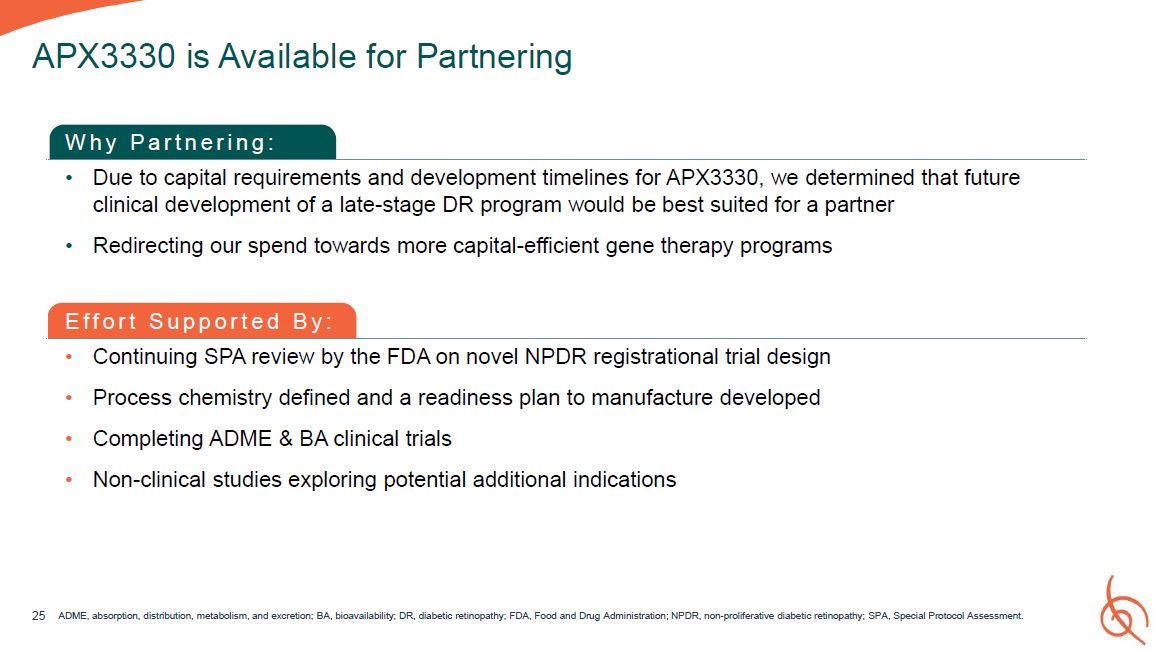
W h y P a r t n e r i n g : Due to capital requirements and development timelines for
APX3330, we determined that future clinical development of a late-stage DR program would be best suited for a partner Redirecting our spend towards more capital-efficient gene therapy programs E f f o r t S u p p o r t e d B y : Continuing
SPA review by the FDA on novel NPDR registrational trial design Process chemistry defined and a readiness plan to manufacture developed Completing ADME & BA clinical trials Non-clinical studies exploring potential additional
indications APX3330 is Available for Partnering 25 ADME, absorption, distribution, metabolism, and excretion; BA, bioavailability; DR, diabetic retinopathy; FDA, Food and Drug Administration; NPDR, non-proliferative diabetic retinopathy;
SPA, Special Protocol Assessment.
A c q u i s i t i o n Combines partnered asset in Phentolamine with Opus’s
cutting-edge, rare IRD gene therapy portfolio Cash runway expected to extend into 2026 Experienced management team U p c o m i n g M i l e s t o n e s * OPGx-LCA5: Ready for enrollment of pediatric subjects in Phase 1/2 study OPGx-BEST1:
Ready to initiate Phase 1/2 clinical trial in Germany OPGx-RHO: IND submission OPGx-RHD12: Ready to initiate NHP GLP tox study Phentolamine Ophthalmic Solution 0.75%: − Dim light disturbances: LYNX-2 Phase 3 topline data expected Q1
2025 − Presbyopia: VEGA-3 Phase 3 topline data expected 1H 2025 Acquisition Creates Leading IRD Franchise with Multiple Near-Term Milestones *All upcoming milestones are subject to regulatory approval. BEST1, bestrophin 1; GLP; Good
Laboratory Practice; IND, Investigational New Drug; IRD, inherited retinal disease; LCA5, Leber congenital amaurosis 5; NHP, nonhuman primate; RDH12, retinol dehydrogenase 12; 26 RHO, rhodopsin.

Every patient’ s eyes tell a story Images of real patients with IRDs.