EXHIBIT 99.1
Published on January 12, 2026
Exhibit 99.1

Delivering on the Promise ofGene Therapy for Rare Inherited Retinal
Diseases January 2026 Nargiza,BEST1 patient

This presentation contains forward-looking statements within the meaning of the
Private Securities Litigation Reform Act of 1995. Such statements include, but are not limited to, statements concerning data from and future enrollment for our clinical trials and our pipeline of additional indications. These forward-looking
statements relate to us, our business prospects and our results of operations and are subject to certain risks and uncertainties posed by many factors and events that could cause our actual business, prospects and results of operations to
differ materially from those anticipated by such forward-looking statements. Factors that could cause or contribute to such differences include, but are not limited to, those described under the heading “Risk Factors” included in our Annual
Report on Form 10-K for the fiscal year ended December 31, 2024 and our Quarterly Reports on Form 10-Q for the fiscal quarters ended March 31, 2025, June 30, 2025 and September 30, 2025 and in our other filings with the U.S. Securities and
Exchange Commission (the “SEC”). Readers are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date of this presentation. In some cases, you can identify forward-looking statements by the
following words: “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “aim,” “may,” “ongoing,” “plan,” “potential,” “predict,” “project,” “should,” “will,” “would” or the negative of these terms or other comparable
terminology, although not all forward-looking statements contain these words. We undertake no obligation to revise any forward-looking statements in order to reflect events or circumstances that might subsequently arise. These forward-looking
statements are based upon our current expectations and involve assumptions that may never materialize or may prove to be incorrect. Actual results and the timing of events could differ materially from those anticipated in such forward-looking
statements as a result of various risks and uncertainties, including, without limitation: our clinical data related to gene therapies for the treatment of inherited retinal diseases is preliminary and related to a relatively small group of
patients, and, as a result, data that initially appears promising may be revised, updated, or invalidated at a later data readout and/or may ultimately not be capable of duplication in additional patients; failure to successfully integrate
our businesses following our acquisition of former Opus Genetics Inc. (the “Opus Acquisition”) could have a material adverse effect on our business, financial condition and results of operations; the Opus Acquisition significantly expanded
our product pipeline and business operations and shifted our business strategies, which may not improve the value of our common stock; our gene therapy product candidates are based on a novel technology that is difficult to develop and
manufacture, which may result in delays and difficulties in obtaining regulatory approval; our planned clinical trials may face substantial delays, result in failure, or provide inconclusive or adverse results that may not satisfy the U.S.
Food and Drug Administration (the “FDA”) requirements to further develop our therapeutic products; delays or difficulties associated with patient enrollment in clinical trials may affect our ability to conduct and complete those clinical
trials and obtain necessary regulatory approvals; changes in regulatory requirements could result in increased costs or delays in development timelines; we depend heavily on the success of our product pipeline; if we fail to find strategic
partners or fail to adequately develop or commercialize our pipeline products, our business will be materially harmed; others may discover, develop, or commercialize products similar to those in our pipeline before or more successfully than
we do or develop generic variants of our products even while our product patents remain active, thereby reducing our market share and potential revenue from product sales; we do not currently have any sales or marketing infrastructure in
place and we have limited drug research and discovery capabilities; the future commercial success of our products could significantly depend upon several uncertain factors, including third-party reimbursement practices and the existence of
competitors with similar products; product liability lawsuits against us or our suppliers or manufacturers could cause us to incur substantial liabilities and could limit commercialization of any product candidate that we may develop; failure
to comply with health and safety laws and regulations could lead to material fines; we have not generated significant revenue from sales of any products and expect to incur losses for the foreseeable future; our future viability is difficult
to assess due to our short operating history and our future need for substantial additional capital, access to which could be limited by any adverse developments that affect the financial services markets; raising additional capital may cause
our stockholders to be diluted, among other adverse effects; we operate in a highly regulated industry and face many challenges adapting to sudden changes in legislative reform or the regulatory environment, which affects our pipeline
stability and could impair our ability to compete in international markets; we may not receive regulatory approval to market our developed product candidates within or outside of the U.S.; with respect to any of our product candidates that
receive marketing approval, we may be subject to substantial penalties if we fail to comply with applicable regulatory requirements; our potential relationships with healthcare providers and third-party payors will be subject to certain
healthcare laws and regulations, which could expose us to extensive potential liabilities; we rely on third parties for material aspects of our business, such as conducting our nonclinical and clinical trials and supplying and manufacturing
bulk drug substances, which exposes us to certain risks; we may be unsuccessful in entering into or maintaining licensing arrangements (such as our license agreement with Viatris, Inc.) or establishing strategic alliances on favorable terms,
which could harm our business; our current focus on the cash-pay utilization for future sales of RYZUMVI may limit our ability to increase sales or achieve profitability with this product; inadequate patent protection for our product
candidates may result in our competitors developing similar or identical products or technology, which would adversely affect our ability to successfully commercialize; we may be unable to obtain full protection for our intellectual property
rights under U.S. or foreign laws; we may become involved in lawsuits for a variety of reasons associated with our intellectual property rights, including alleged infringement suits initiated by third parties; we are dependent on our key
personnel, and if we are not successful in attracting and retaining highly qualified personnel, we may not be able to successfully implement our business strategy; as we grow, we may not be able to operate internationally or adequately
develop and expand our sales, marketing, distribution, and other corporate functions, which could disrupt our operations; the market price of our common stock is expected to be volatile; our common stock may be subject to delisting from the
Nasdaq Capital Market, which could adversely affect our ability to access capital markets; factors out of our control related to our securities, such as securities litigation or actions of activist stockholders, could adversely affect our
business and stock price and cause us to incur significant expenses; our business could experience an adverse impact from current or proposed tariffs on imported goods we purchase; our ability to utilize our common stock to finance future
capital needs, or for other purposes, is limited by our authorized shares available for issuance; and instability and operational disruptions at government agencies, such as the FDA, may adversely impact our development and commercialization
plans by causing delays and requiring the use of additional, unforeseen resources to obtain regulatory approval for trials or products in our pipeline. The foregoing review of important factors that could cause actual events to differ from
expectations should not be construed as exhaustive. Readers are urged to carefully review and consider the various disclosures made by us in this report and in our other reports filed with the SEC that advise interested parties of the risks
and factors that may affect our business. All forward-looking statements contained in this presentation speak only as of the date on which they were made. We undertake no obligation to update such statements to reflect events that occur or
circumstances that exist after the date on which they were made. Disclosures and Forward-Looking Statements 2
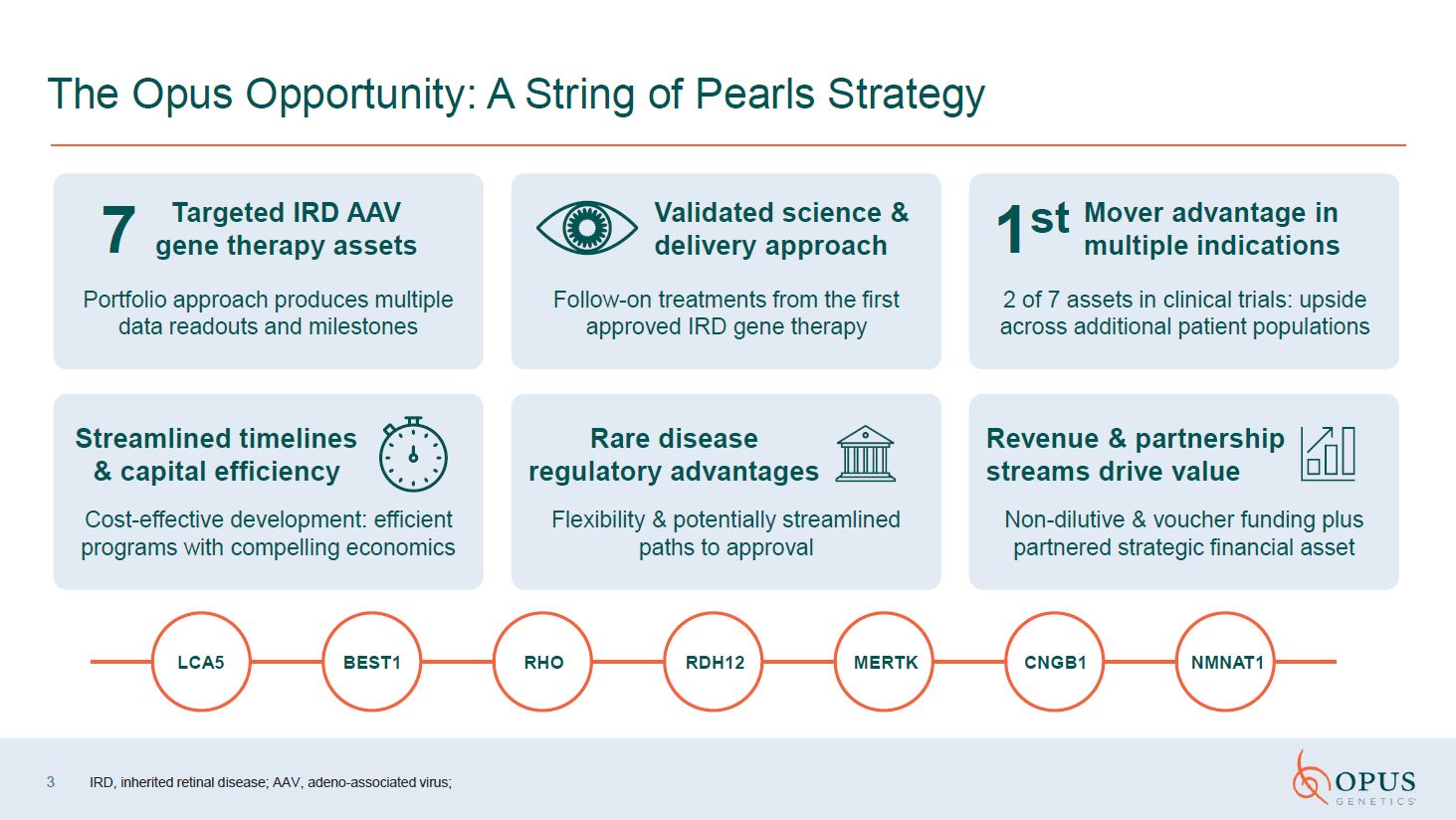
The Opus Opportunity: A String of Pearls
Strategy LCA5 RHO RDH12 CNGB1 NMNAT1 MERTK BEST1 Portfolio approach produces multiple data readouts and milestones Follow-on treatments from the first approved IRD gene therapy 2 of 7 assets in clinical trials: upside across
additional patient populations Cost-effective development: efficient programs with compelling economics Flexibility & potentially streamlined paths to approval Non-dilutive & voucher funding plus partnered strategic financial
asset Validated science & delivery approach 7 Targeted IRD AAV gene therapy assets Mover advantage in multiple indications 1st Streamlined timelines & capital efficiency Rare disease regulatory advantages Revenue &
partnership streams drive value 3 IRD, inherited retinal disease; AAV, adeno-associated virus;
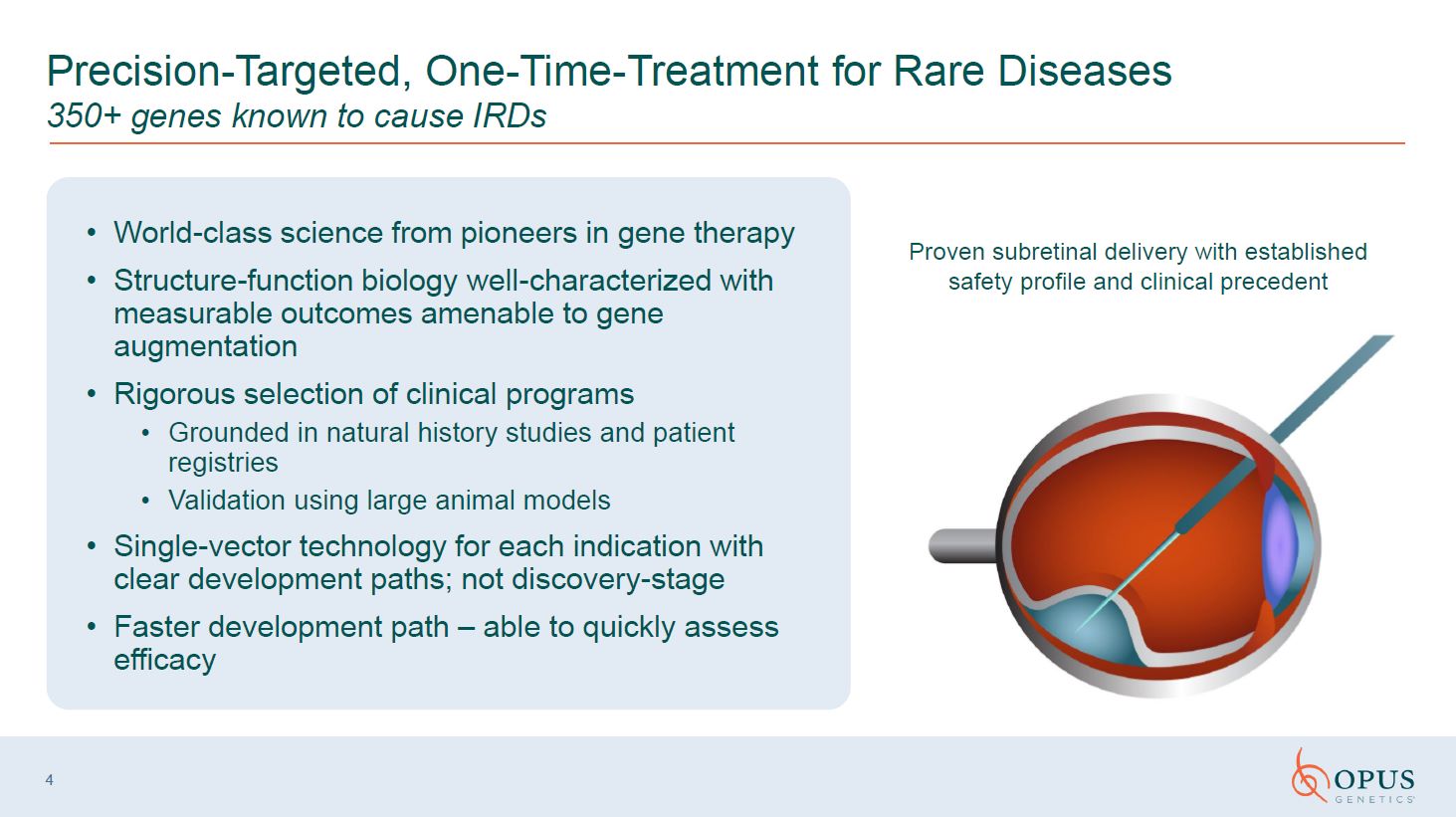
4 Precision-Targeted, One-Time-Treatment for Rare Diseases 350+ genes known to
cause IRDs Proven subretinal delivery with established safety profile and clinical precedent World-class science from pioneers in gene therapy Structure-function biology well-characterized with measurable outcomes amenable to gene
augmentation Rigorous selection of clinical programs Grounded in natural history studies and patient registries Validation using large animal models Single-vector technology for each indication with clear development paths; not
discovery-stage Faster development path – able to quickly assess efficacy
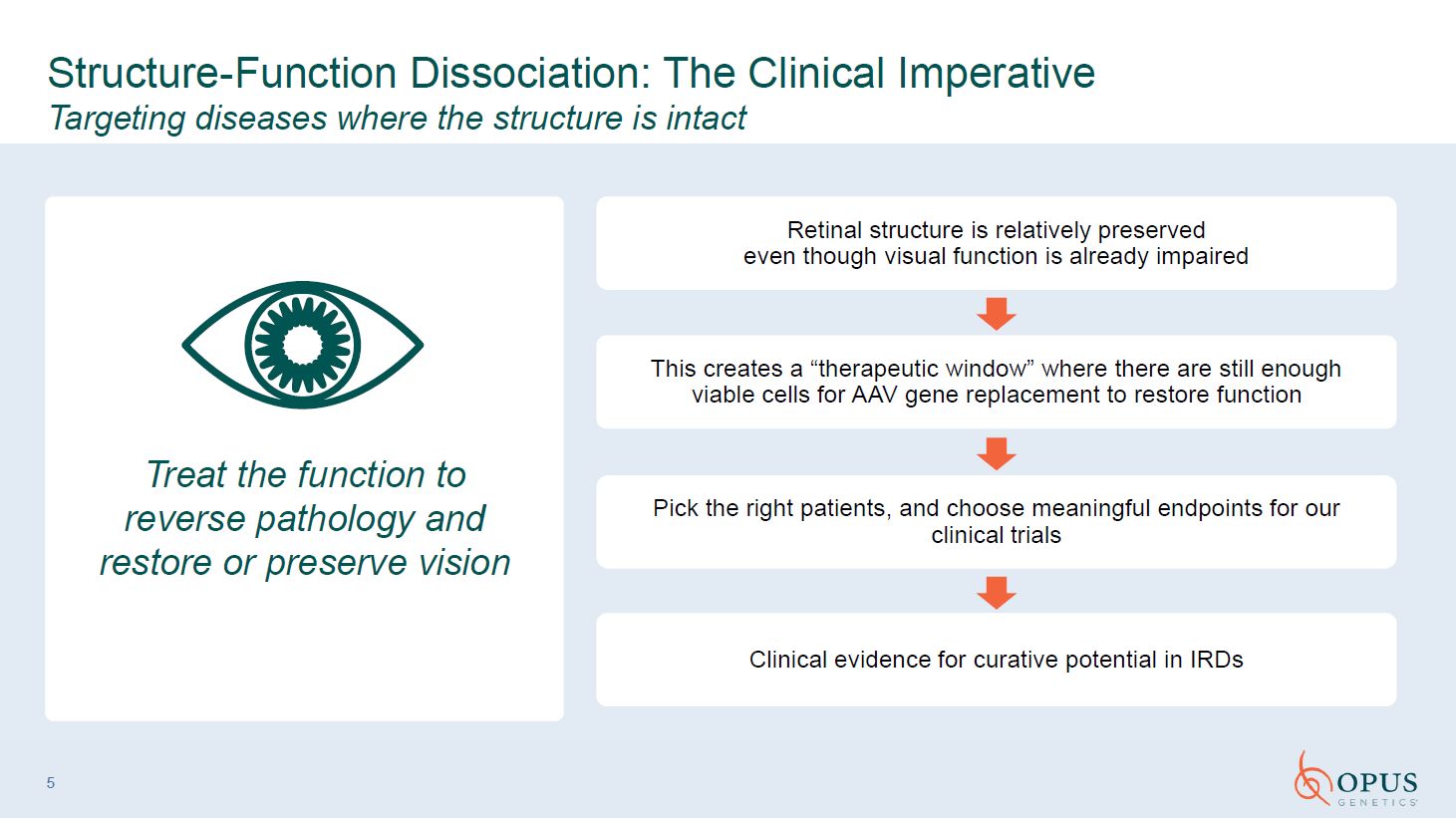
5 Treat the function to reverse pathology and restore or preserve
vision Structure-Function Dissociation: The Clinical Imperative Targeting diseases where the structure is intact Retinal structure is relatively preservedeven though visual function is already impaired This creates a “therapeutic window”
where there are still enough viable cells for AAV gene replacement to restore function Pick the right patients, and choose meaningful endpoints for our clinical trials Clinical evidence for curative potential in IRDs
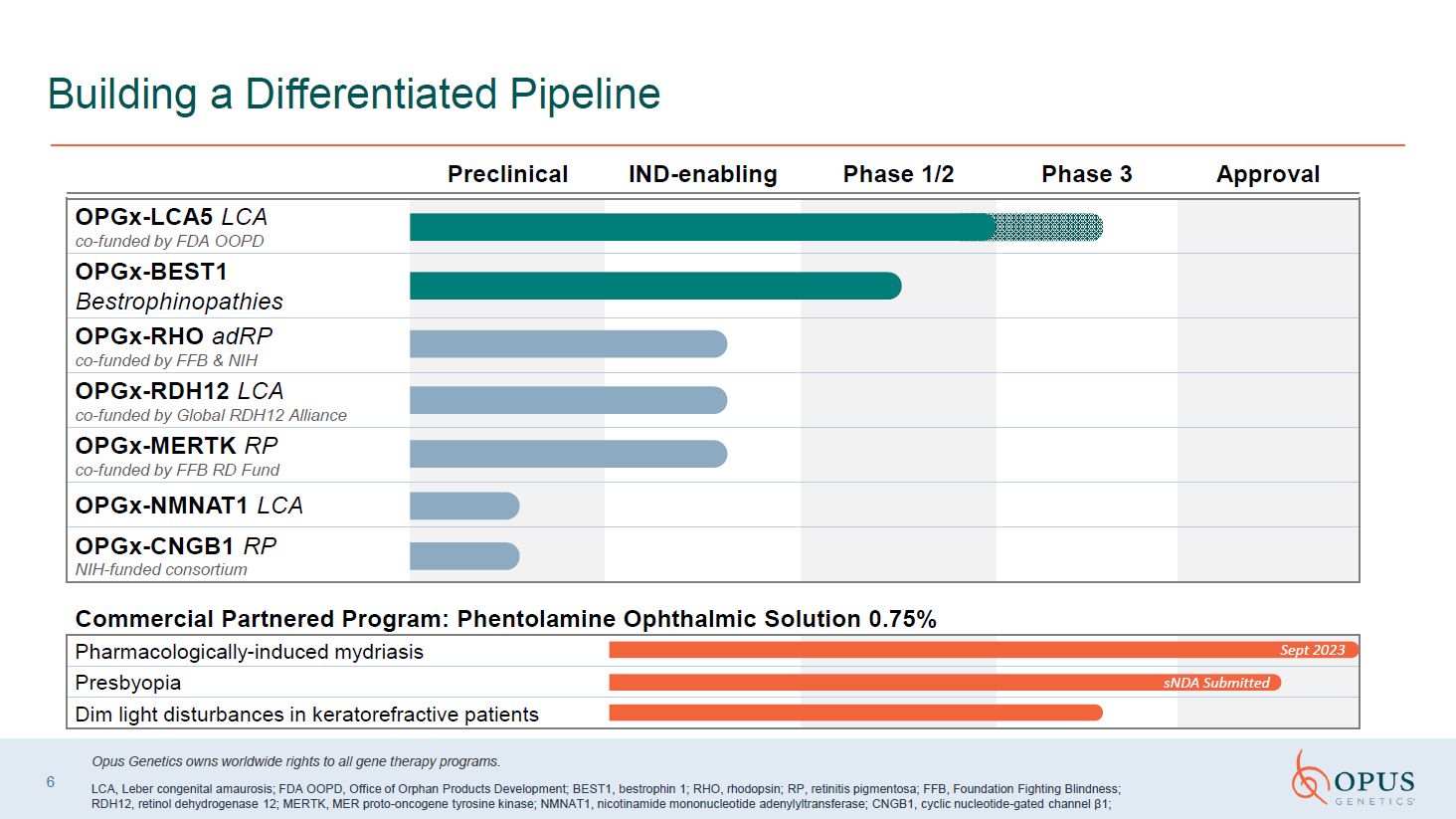
Building a Differentiated Pipeline OPGx-LCA5 LCA co-funded by FDA
OOPD OPGx-BEST1 Bestrophinopathies OPGx-RHO adRP co-funded by FFB & NIH OPGx-RDH12 LCA co-funded by Global RDH12 Alliance OPGx-MERTK RP co-funded by FFB RD Fund OPGx-NMNAT1 LCA OPGx-CNGB1 RP NIH-funded consortium Commercial
Partnered Program: Phentolamine Ophthalmic Solution 0.75% Pharmacologically-induced mydriasis Presbyopia Dim light disturbances in keratorefractive patients sNDA Submitted 6 Preclinical IND-enabling Phase 1/2 Phase 3 Approval Opus
Genetics owns worldwide rights to all gene therapy programs. LCA, Leber congenital amaurosis; FDA OOPD, Office of Orphan Products Development; BEST1, bestrophin 1; RHO, rhodopsin; RP, retinitis pigmentosa; FFB, Foundation Fighting Blindness;
RDH12, retinol dehydrogenase 12; MERTK, MER proto-oncogene tyrosine kinase; NMNAT1, nicotinamide mononucleotide adenylyltransferase; CNGB1, cyclic nucleotide-gated channel β1; Sept 2023
7 Actively Advancing Lead Indications OPGx-LCA5 Positive Phase 1/2 safety and
efficacy results observed in adult and pediatric participants Successful Type B RMAT meeting Enrollment ongoing in run-in period for planned, adaptive Phase 3 trial Multiple Regulatory Designations: Rare Pediatric Disease Regenerative
Medicine Advanced Therapy Orphan Drug Potential eligibility for Priority Review Voucher upon BLA approval OPGx-BEST1 Phase 1/2 trial ongoing; first participant dosed in Q4 2025 IDMC completed pre-specified safety review of the
one-month data from the sentinel participant; recommended continued enrollment without modification Initial data expected in Q1 2026; 3-month results from entire Cohort 1 expected in mid-2026 Potential treatment for both the dominant and
recessive forms of BEST1 disease Potentially eligible for multiple regulatory designations Early-onset, severe hereditary retinal degeneration BEST1-related mutations associated with retinal degenerative diseases
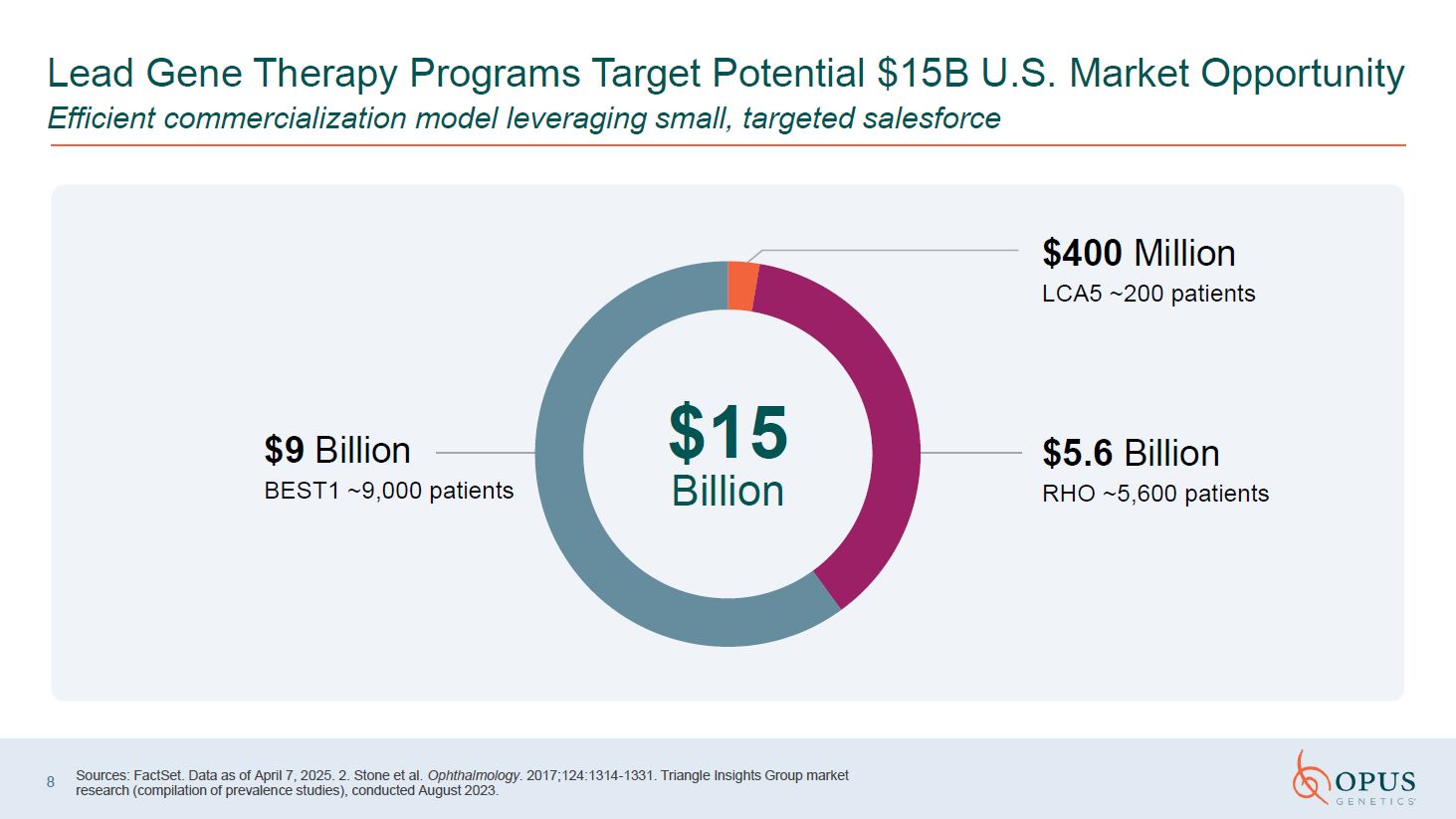
8 Sources: FactSet. Data as of April 7, 2025. 2. Stone et al. Ophthalmology.
2017;124:1314-1331. Triangle Insights Group market research (compilation of prevalence studies), conducted August 2023. $400 Million LCA5 ~200 patients $5.6 Billion RHO ~5,600 patients $9 Billion BEST1 ~9,000 patients $15 Billion
Lead Gene Therapy Programs Target Potential $15B U.S. Market Opportunity Efficient commercialization model leveraging small, targeted salesforce

OPGx-BEST1 Juan,BEST1 patient
BEST1 Mutations are Associated with Retinal Degeneration 1. Triangle Insights
Group market research (compilation of prevalence studies), conducted August 2023. 2. Amato A, et al. Saudi J Ophthalmol. 2023;37(4):287-295. 3. Johnson AA, et al. Prog Retin Eye Res. 2017;58:45-69. 4. Tripathy K, et al. StatPearls [Internet].
Treasure Island (FL): StatPearls Publishing; 2024. OPGx-BEST1 Designed to restore retinal ion homeostasis in bestrophinopathies, ameliorating retinal structural and functional deficits Targeted using the AAV2 capsid employed in Luxturna
and an RPE-specific promoter 10 Prevalence ~9,000 patients the U.S.1 Accounts for ~3.5% of all IRDs2 Clinical Characteristics Mutations in BEST1 have been associated with at least five clinically distinct retinal degenerative diseases3
Bestrophinopathy is characterized by retinal lesions, with symptoms including dimness of vision, metamorphopsia (distorted vision), or scotoma (blind spot)4 Mutations, depending on their impact on BEST1 function, may lead to serous retinal
detachment, vitelliform lesions in the macular region, macular atrophy, and loss of central vision Most bestrophinopathies exhibit a slow rate of decline and central photoreceptors usually remain viable for decades, providing a wide
therapeutic window
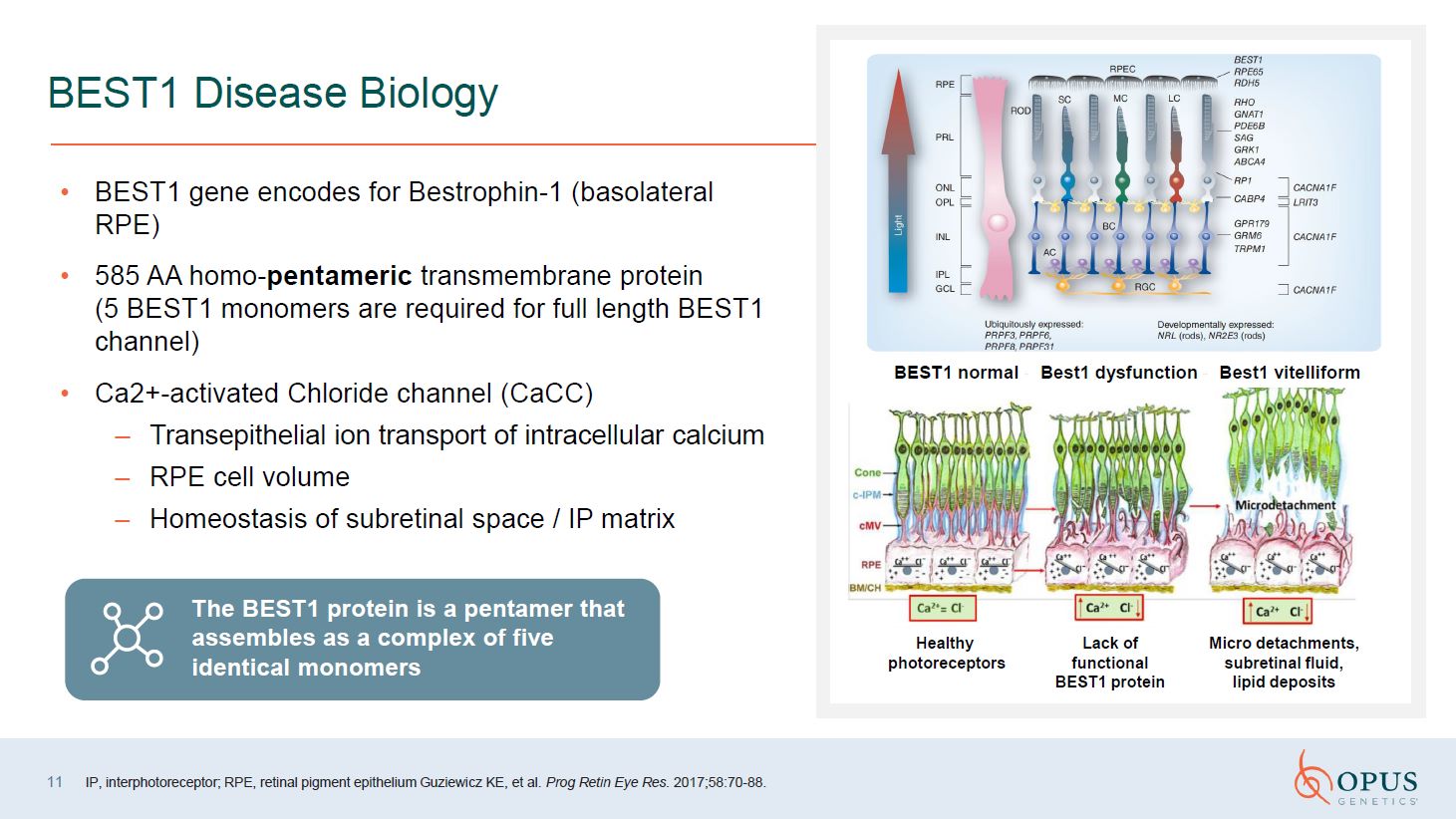
11 BEST1 Disease Biology BEST1 gene encodes for Bestrophin-1 (basolateral
RPE) 585 AA homo-pentameric transmembrane protein (5 BEST1 monomers are required for full length BEST1 channel) Ca2+-activated Chloride channel (CaCC) Transepithelial ion transport of intracellular calcium RPE cell volume Homeostasis of
subretinal space / IP matrix IP, interphotoreceptor; RPE, retinal pigment epithelium Guziewicz KE, et al. Prog Retin Eye Res. 2017;58:70-88. Best1 vitelliform Best1 dysfunction BEST1 normal Healthy photoreceptors Lack of functional
BEST1 protein Micro detachments, subretinal fluid, lipid deposits The BEST1 protein is a pentamer that assembles as a complex of five identical monomers
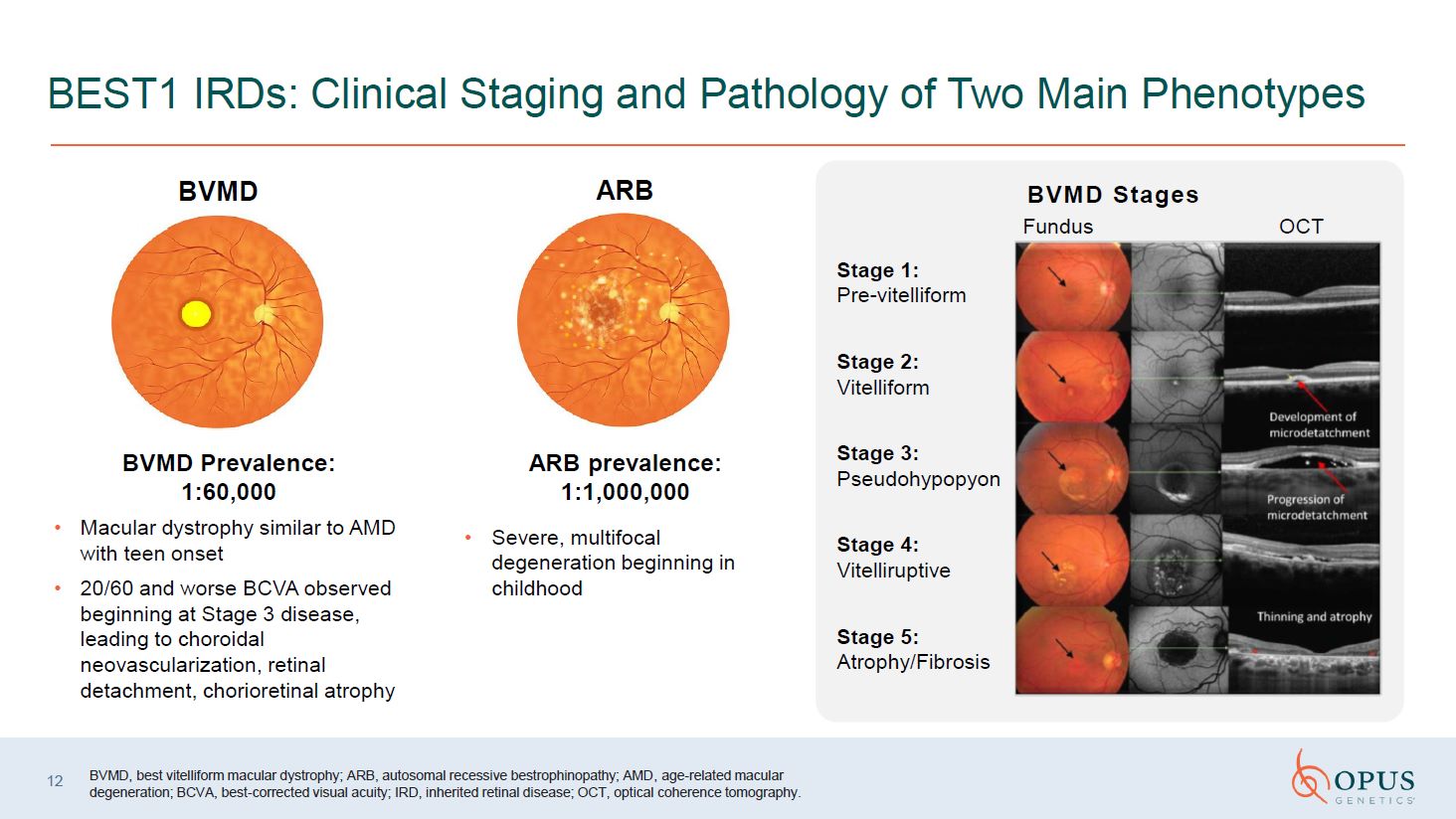
12 BEST1 IRDs: Clinical Staging and Pathology of Two Main
Phenotypes BVMD BVMD Prevalence: 1:60,000 Macular dystrophy similar to AMD with teen onset 20/60 and worse BCVA observed beginning at Stage 3 disease, leading to choroidal neovascularization, retinal detachment, chorioretinal
atrophy BVMD, best vitelliform macular dystrophy; ARB, autosomal recessive bestrophinopathy; AMD, age-related macular degeneration; BCVA, best-corrected visual acuity; IRD, inherited retinal disease; OCT, optical coherence
tomography. Fundus OCT Stage 1: Pre-vitelliform Stage 2: Vitelliform Stage 3: Pseudohypopyon Stage 4: Vitelliruptive Stage 5: Atrophy/Fibrosis BVMD Stages ARB ARB prevalence: 1:1,000,000 Severe, multifocal degeneration
beginning in childhood
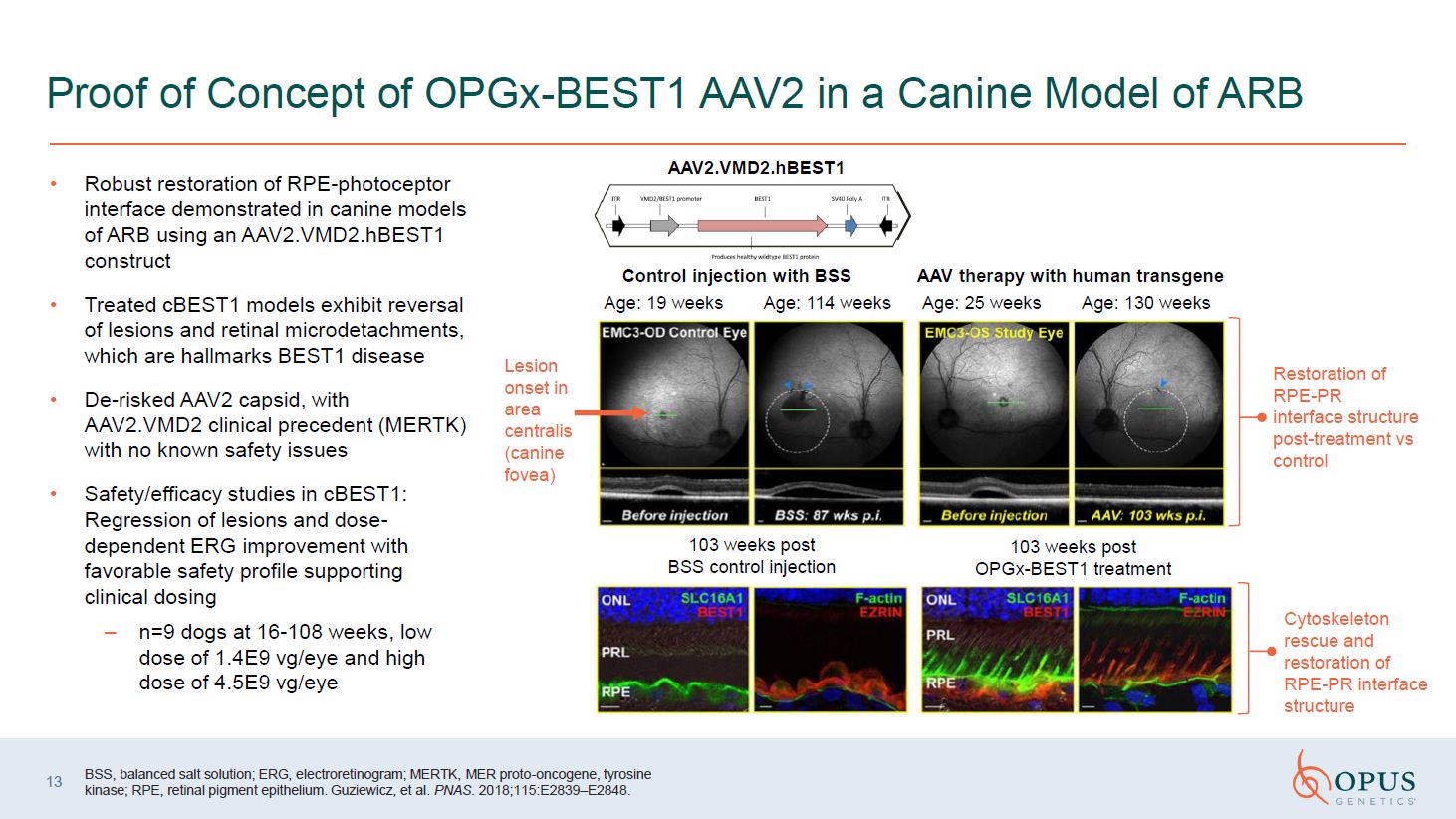
13 Proof of Concept of OPGx-BEST1 AAV2 in a Canine Model of ARB BSS, balanced
salt solution; ERG, electroretinogram; MERTK, MER proto-oncogene, tyrosine kinase; RPE, retinal pigment epithelium. Guziewicz, et al. PNAS. 2018;115:E2839–E2848. Robust restoration of RPE-photoceptor interface demonstrated in canine models
of ARB using an AAV2.VMD2.hBEST1 construct Treated cBEST1 models exhibit reversal of lesions and retinal microdetachments, which are hallmarks BEST1 disease De-risked AAV2 capsid, with AAV2.VMD2 clinical precedent (MERTK) with no known
safety issues Safety/efficacy studies in cBEST1: Regression of lesions and dose-dependent ERG improvement with favorable safety profile supporting clinical dosing n=9 dogs at 16-108 weeks, low dose of 1.4E9 vg/eye and high dose of 4.5E9
vg/eye 103 weeks post BSS control injection 103 weeks post OPGx-BEST1 treatment Cytoskeleton rescue and restoration of RPE-PR interface structure Control injection with BSS AAV therapy with human transgene Age: 19 weeks Age: 114
weeks Age: 25 weeks Age: 130 weeks Restoration of RPE-PR interface structure post-treatment vs control Lesion onset in area centralis (canine fovea) AAV2.VMD2.hBEST1
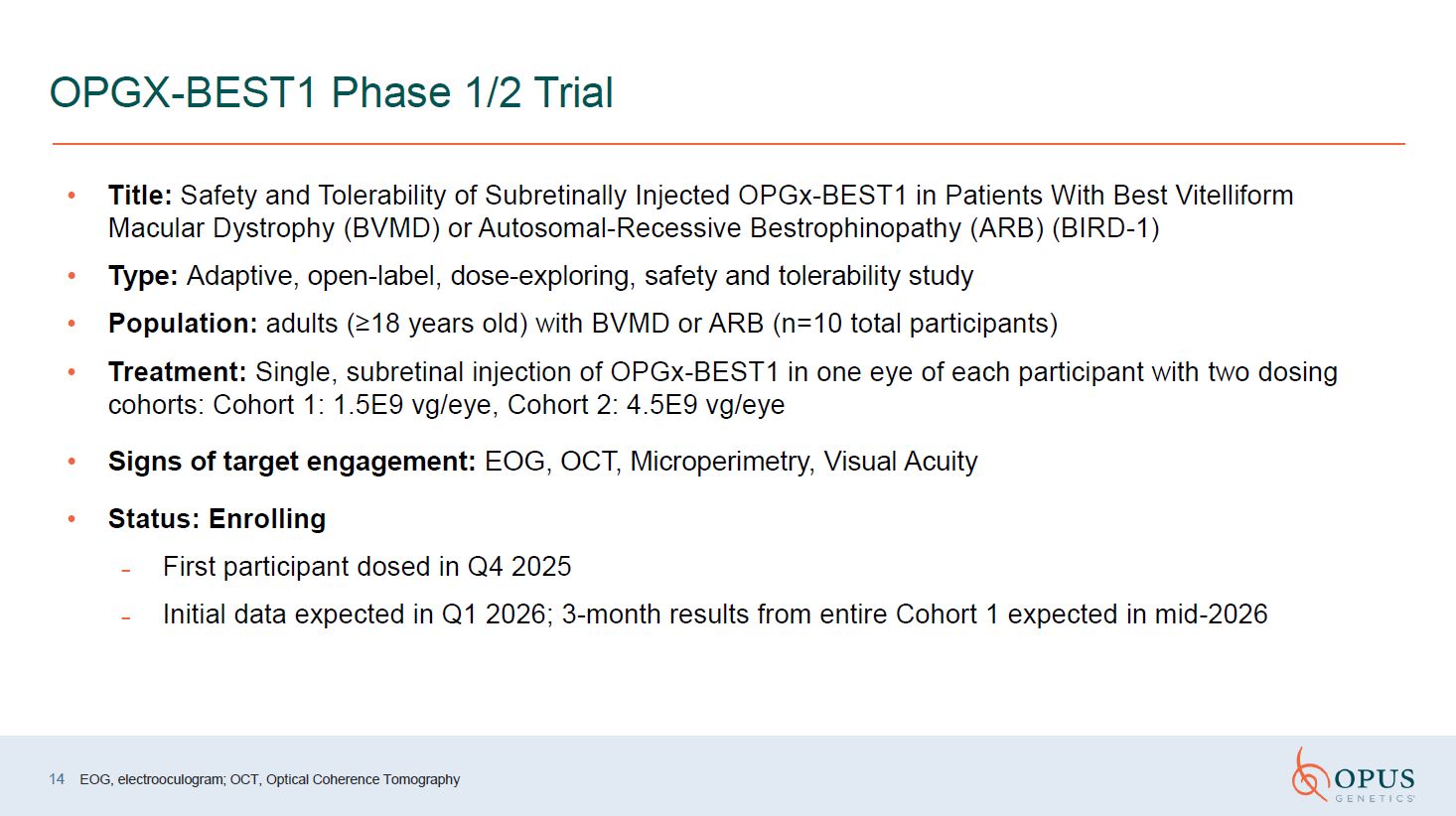
OPGX-BEST1 Phase 1/2 Trial Title: Safety and Tolerability of Subretinally
Injected OPGx-BEST1 in Patients With Best Vitelliform Macular Dystrophy (BVMD) or Autosomal-Recessive Bestrophinopathy (ARB) (BIRD-1) Type: Adaptive, open-label, dose-exploring, safety and tolerability study Population: adults (≥18 years
old) with BVMD or ARB (n=10 total participants) Treatment: Single, subretinal injection of OPGx-BEST1 in one eye of each participant with two dosing cohorts: Cohort 1: 1.5E9 vg/eye, Cohort 2: 4.5E9 vg/eye Signs of target engagement: EOG,
OCT, Microperimetry, Visual Acuity Status: Enrolling First participant dosed in Q4 2025 Initial data expected in Q1 2026; 3-month results from entire Cohort 1 expected in mid-2026 14 EOG, electrooculogram; OCT, Optical Coherence
Tomography

OPGx-LCA5 Alan,LCA5 patient
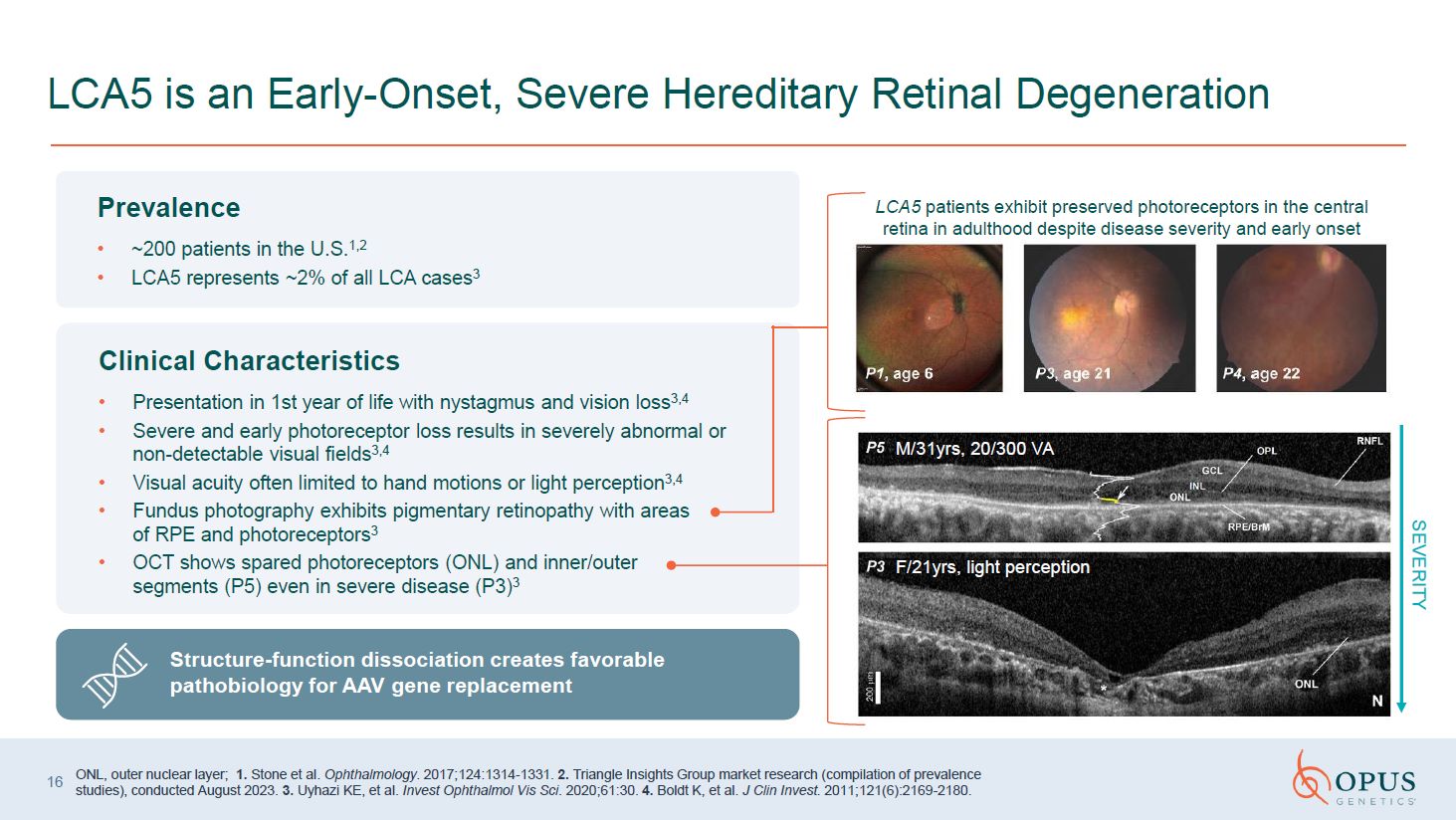
Prevalence ~200 patients in the U.S.1,2 LCA5 represents ~2% of all LCA
cases3 Clinical Characteristics Presentation in 1st year of life with nystagmus and vision loss3,4 Severe and early photoreceptor loss results in severely abnormal or non-detectable visual fields3,4 Visual acuity often limited to hand
motions or light perception3,4 Fundus photography exhibits pigmentary retinopathy with areas of RPE and photoreceptors3 OCT shows spared photoreceptors (ONL) and inner/outer segments (P5) even in severe disease (P3)3 LCA5 is an
Early-Onset, Severe Hereditary Retinal Degeneration SEVERITY M/31yrs, 20/300 VA F/21yrs, light perception 16 LCA5 patients exhibit preserved photoreceptors in the central retina in adulthood despite disease severity and early
onset ONL, outer nuclear layer; 1. Stone et al. Ophthalmology. 2017;124:1314-1331. 2. Triangle Insights Group market research (compilation of prevalence studies), conducted August 2023. 3. Uyhazi KE, et al. Invest Ophthalmol Vis Sci.
2020;61:30. 4. Boldt K, et al. J Clin Invest. 2011;121(6):2169-2180. Structure-function dissociation creates favorable pathobiology for AAV gene replacement
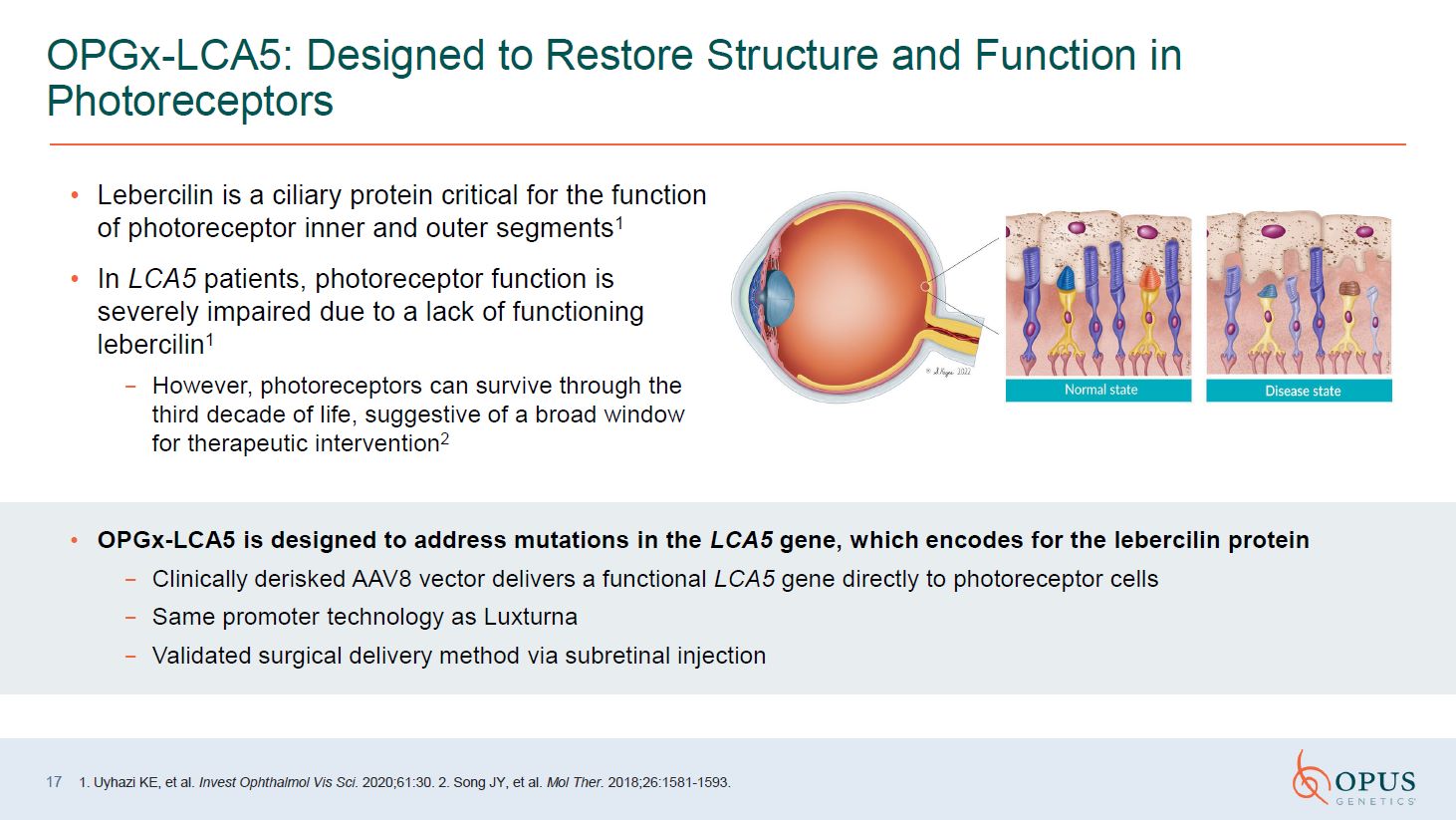
Lebercilin is a ciliary protein critical for the function of photoreceptor inner
and outer segments1 In LCA5 patients, photoreceptor function is severely impaired due to a lack of functioning lebercilin1 However, photoreceptors can survive through the third decade of life, suggestive of a broad window for therapeutic
intervention2 17 OPGx-LCA5: Designed to Restore Structure and Function in Photoreceptors 1. Uyhazi KE, et al. Invest Ophthalmol Vis Sci. 2020;61:30. 2. Song JY, et al. Mol Ther. 2018;26:1581-1593. OPGx-LCA5 is designed to address
mutations in the LCA5 gene, which encodes for the lebercilin protein Clinically derisked AAV8 vector delivers a functional LCA5 gene directly to photoreceptor cells Same promoter technology as Luxturna Validated surgical delivery method
via subretinal injection
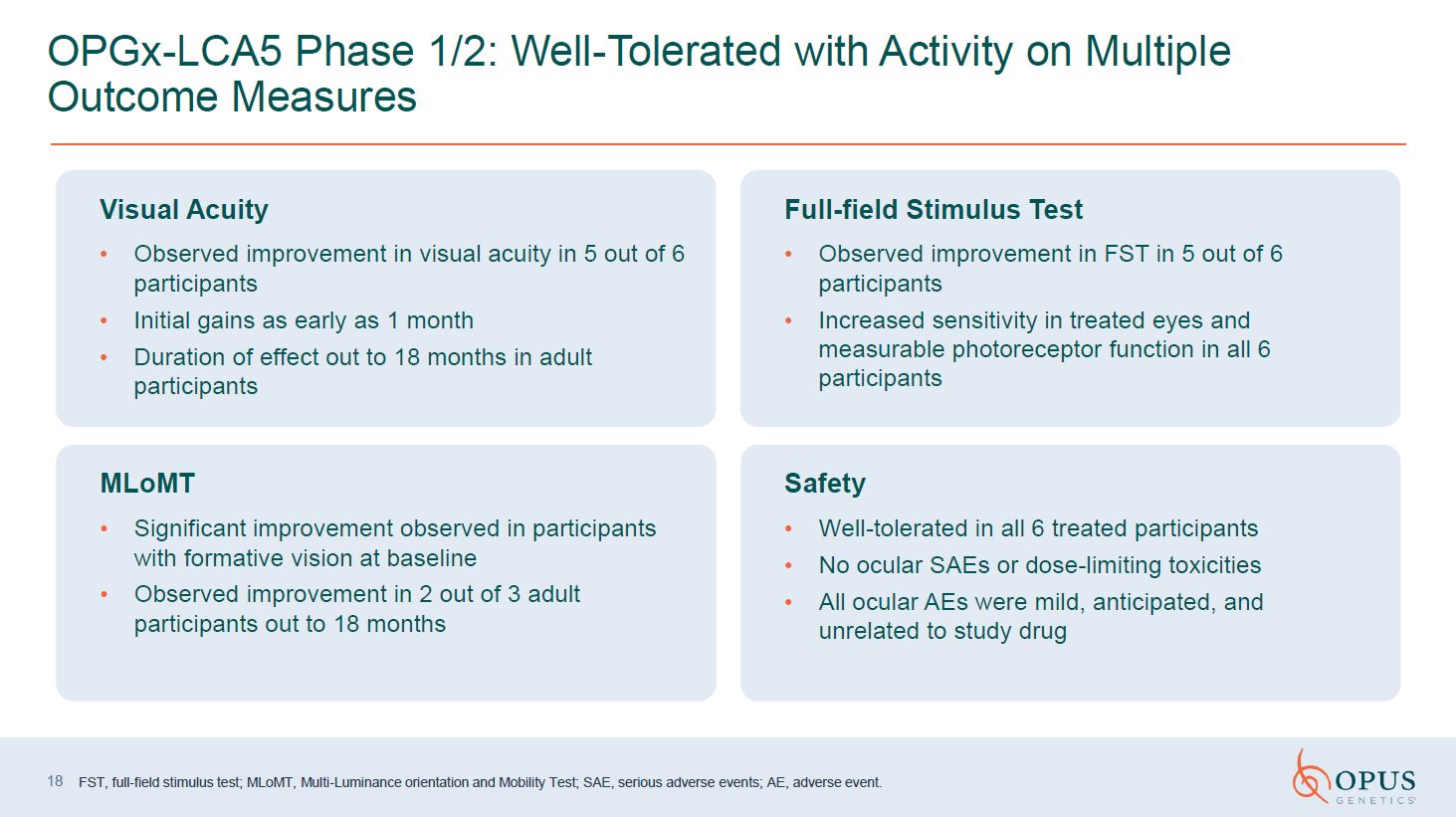
OPGx-LCA5 Phase 1/2: Well-Tolerated with Activity on Multiple Outcome
Measures FST, full-field stimulus test; MLoMT, Multi-Luminance orientation and Mobility Test; SAE, serious adverse events; AE, adverse event. 18 Visual Acuity Observed improvement in visual acuity in 5 out of 6 participants Initial gains
as early as 1 month Duration of effect out to 18 months in adult participants Full-field Stimulus Test Observed improvement in FST in 5 out of 6 participants Increased sensitivity in treated eyes and measurable photoreceptor function in
all 6 participants MLoMT Significant improvement observed in participants with formative vision at baseline Observed improvement in 2 out of 3 adult participants out to 18 months Safety Well-tolerated in all 6 treated participants No
ocular SAEs or dose-limiting toxicities All ocular AEs were mild, anticipated, and unrelated to study drug
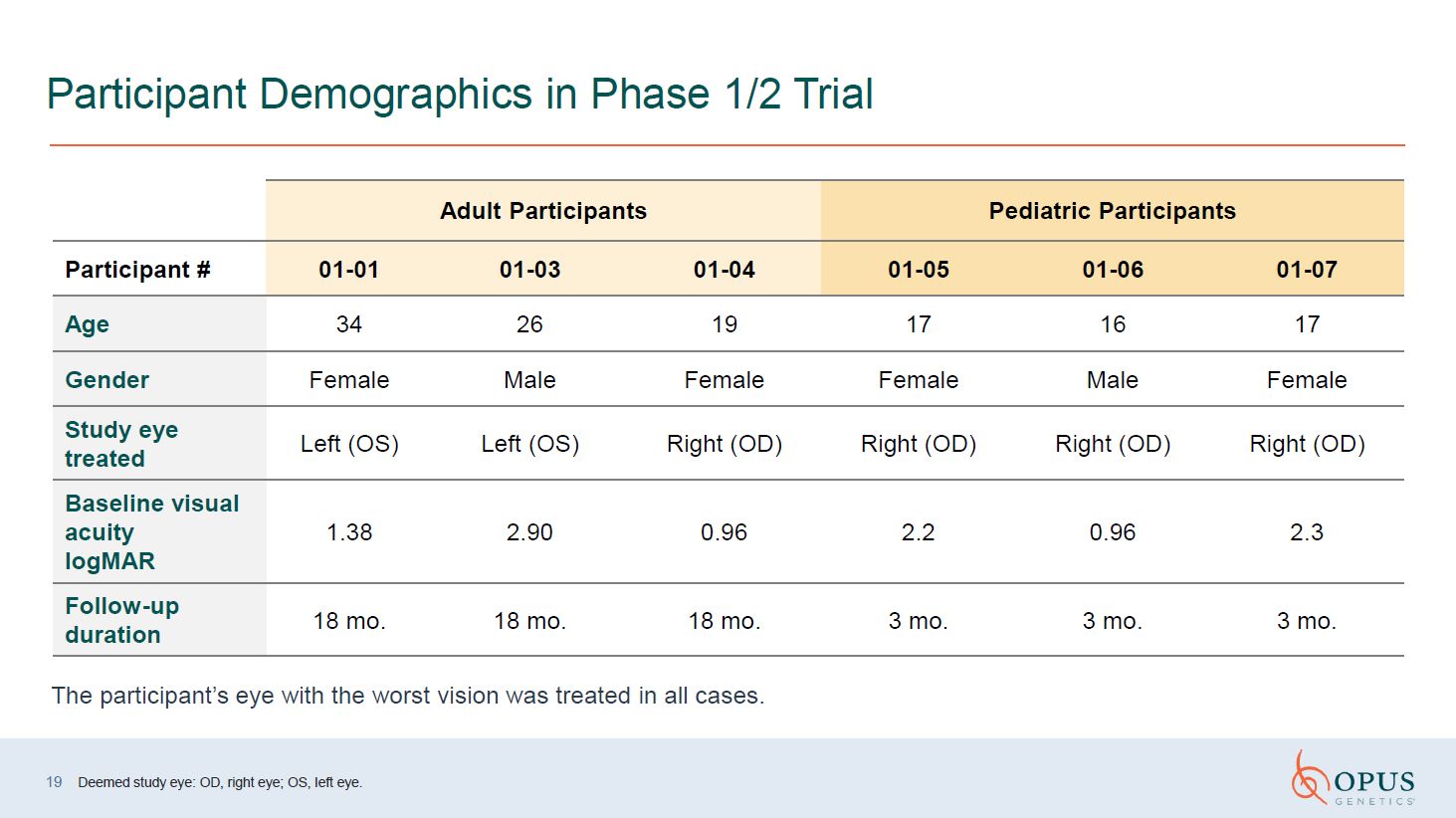
Participant Demographics in Phase 1/2 Trial Deemed study eye: OD, right eye;
OS, left eye. Adult Participants Pediatric Participants Participant # 01-01 01-03 01-04 01-05 01-06 01-07 Age 34 26 19 17 16 17 Gender Female Male Female Female Male Female Study eye treated Left (OS) Left
(OS) Right (OD) Right (OD) Right (OD) Right (OD) Baseline visual acuity logMAR 1.38 2.90 0.96 2.2 0.96 2.3 Follow-up duration 18 mo. 18 mo. 18 mo. 3 mo. 3 mo. 3 mo. The participant’s eye with the worst vision was treated
in all cases. 19
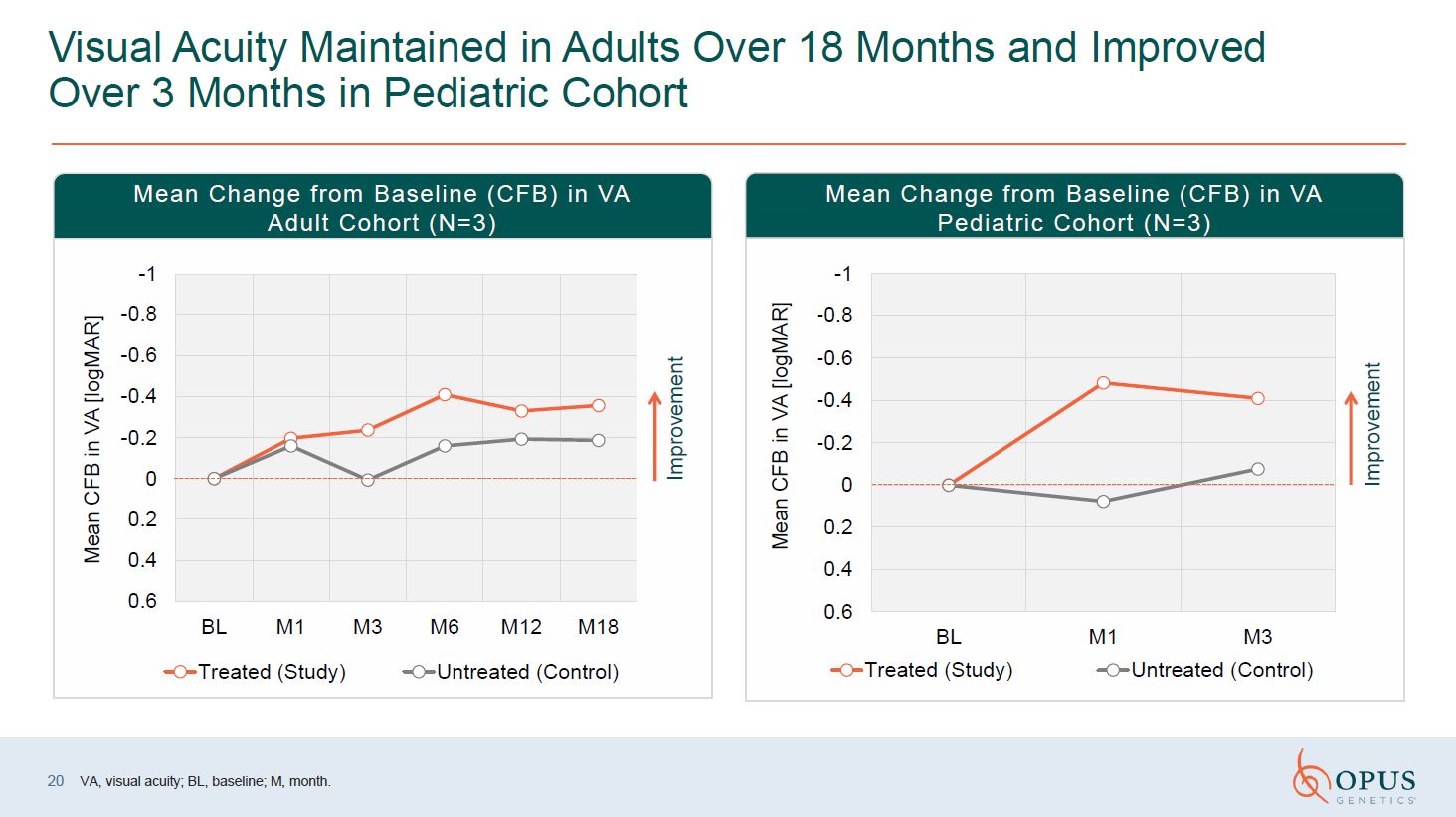
Visual Acuity Maintained in Adults Over 18 Months and Improved Over 3 Months in
Pediatric Cohort Improvement Improvement Mean Change from Baseline (CFB) in VA Adult Cohort (N=3) Mean Change from Baseline (CFB) in VA Pediatric Cohort (N=3) VA, visual acuity; BL, baseline; M, month. 20
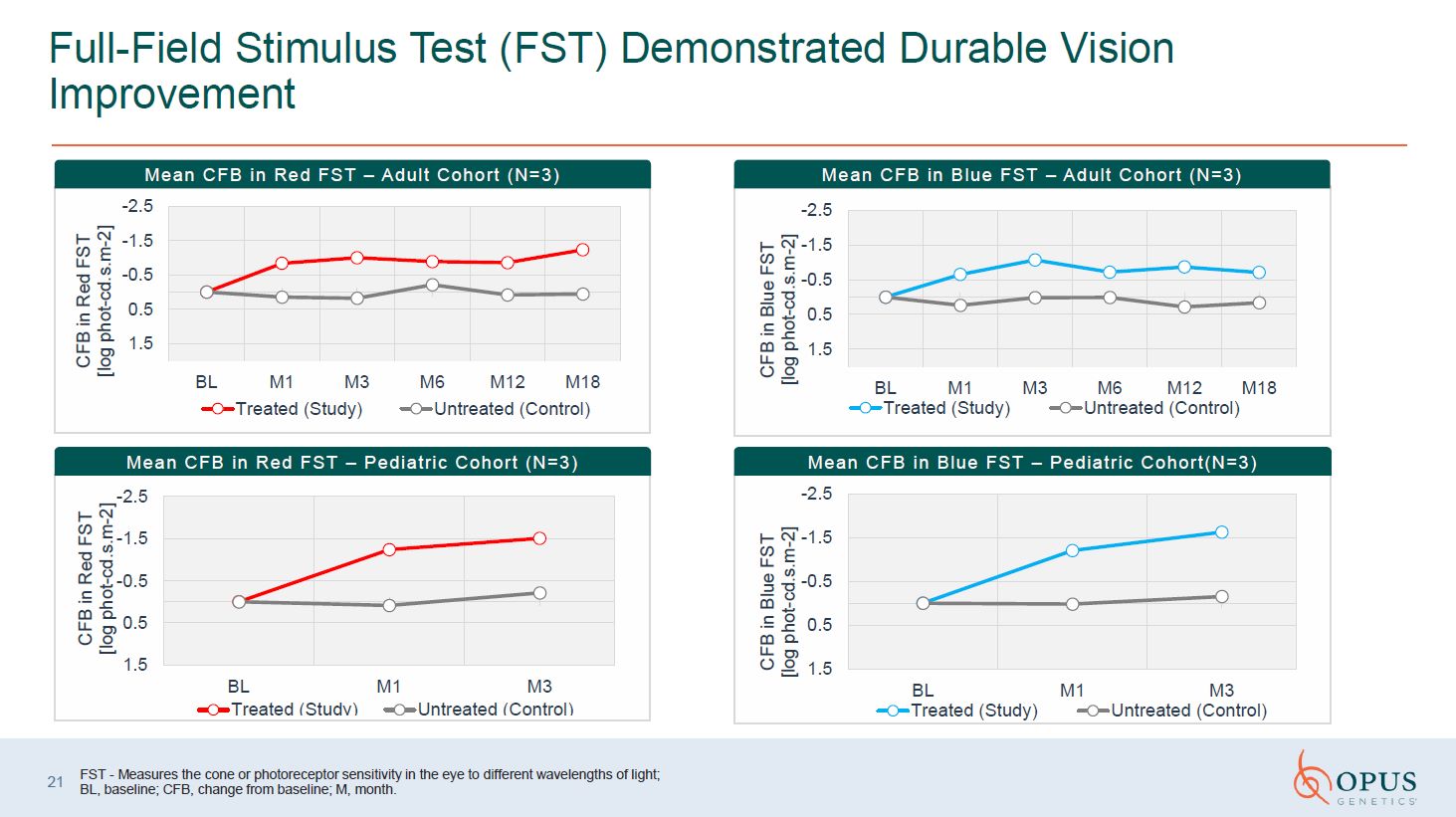
Full-Field Stimulus Test (FST) Demonstrated Durable Vision Improvement Mean CFB
in Red FST – Adult Cohort (N=3) Mean CFB in Blue FST – Adult Cohort (N=3) Mean CFB in Red FST – Pediatric Cohort (N=3) Mean CFB in Blue FST – Pediatric Cohort(N=3) FST - Measures the cone or photoreceptor sensitivity in the eye to
different wavelengths of light; BL, baseline; CFB, change from baseline; M, month. 21
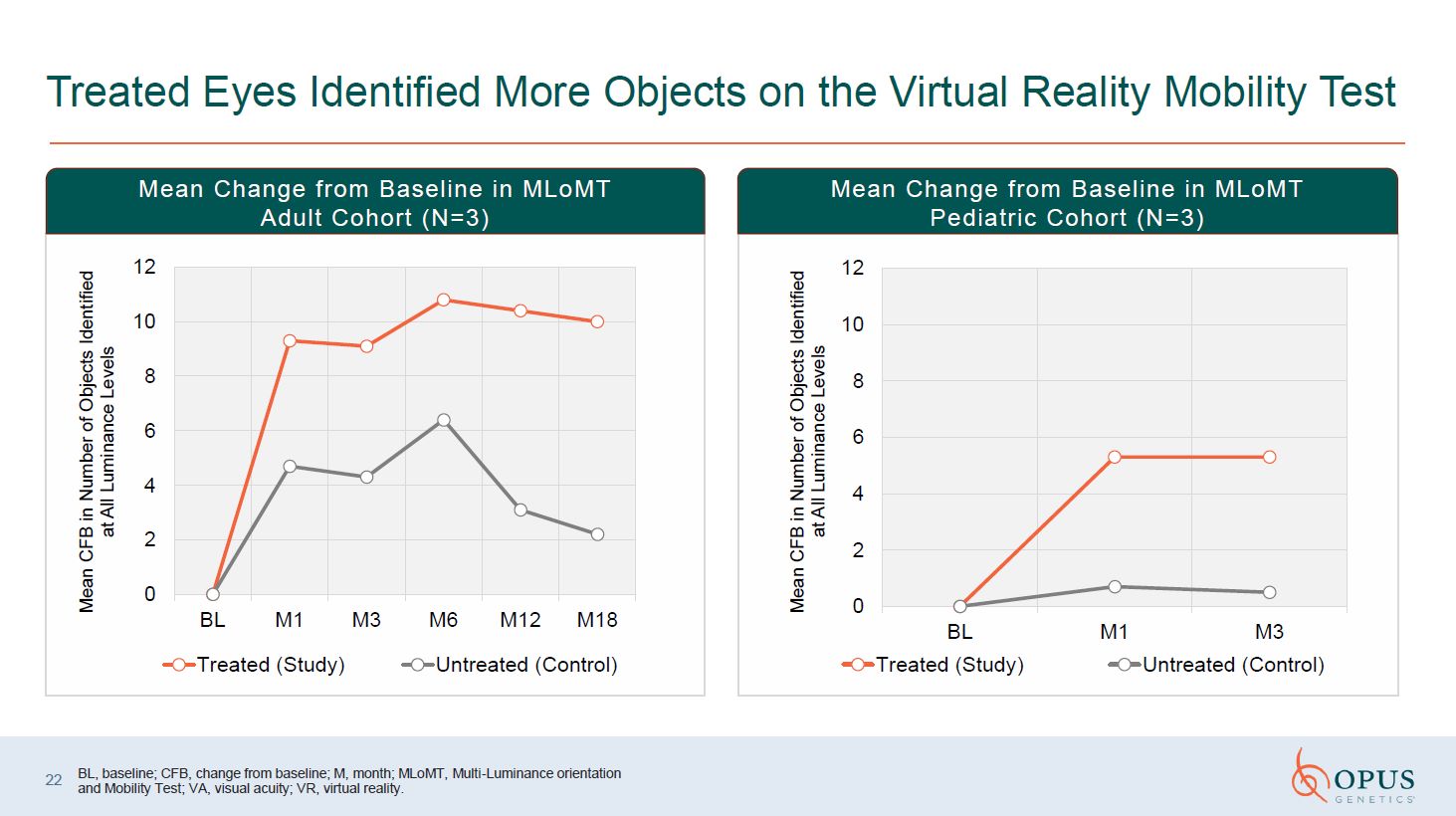
Treated Eyes Identified More Objects on the Virtual Reality Mobility Test Mean
Change from Baseline in MLoMT Adult Cohort (N=3) Mean Change from Baseline in MLoMT Pediatric Cohort (N=3) BL, baseline; CFB, change from baseline; M, month; MLoMT, Multi-Luminance orientation and Mobility Test; VA, visual acuity; VR,
virtual reality. 22
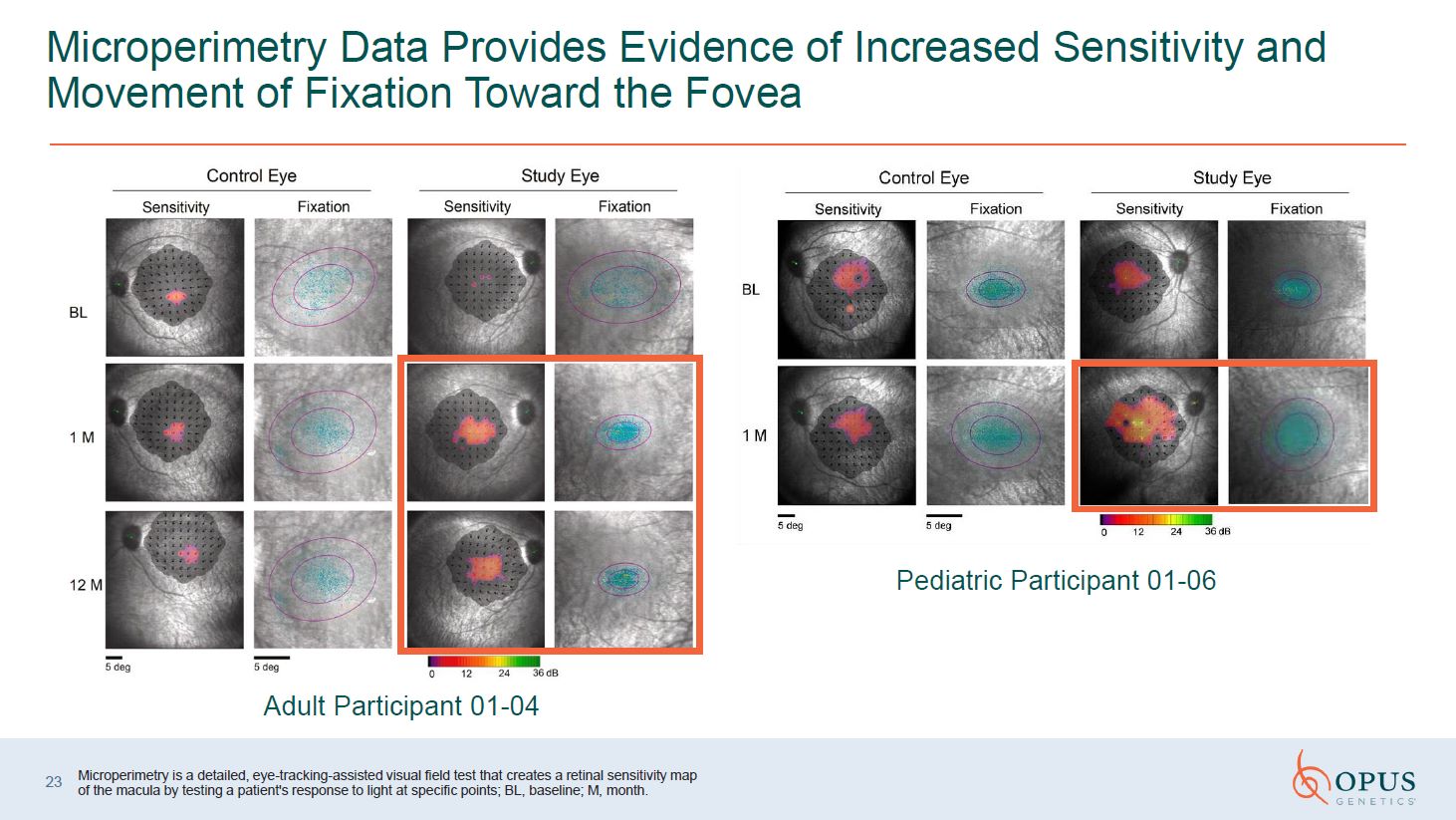
Microperimetry Data Provides Evidence of Increased Sensitivity and Movement of
Fixation Toward the Fovea Adult Participant 01-04 Pediatric Participant 01-06 Microperimetry is a detailed, eye-tracking-assisted visual field test that creates a retinal sensitivity map of the macula by testing a patient's response to
light at specific points; BL, baseline; M, month. 23
OPGx-LCA5 Program Positioned for Rapid Advancement 24 BLA, Biologics License
Application; *Potential PRV opportunities contingent on timing of FDA approval and/or congressional reauthorization of PRV program Excellent safety data in all participants with follow-up out to 18 months in adult cohort Robust biologic
activity corroborated through multiple functional outcomes: VA and FST improvements in 5 out of 6 participants suggest enhanced visual perception and clarity Improvement in MLoMT translates to potential improved ability to navigate the
environment and perform daily activities Rare Pediatric Disease, Orphan Drug and Regenerative Medicine Advanced Therapy designations received from the FDA; potential eligibility for Priority Review Voucher upon BLA approval* Successful
Type B RMAT meeting completed with enrollment ongoing in run-in period for planned, adaptive Phase 3 trial

Partnered Asset: Phentolamine Ophthalmic Solution 0.75%
1 APPROVED: Pharmacologically-induced mydriasis1 100M eye dilations conducted
every year2 2 3 sNDA SUBMITTED: Presbyopia Progressive loss of ability to focus on close objects results in blurred near vision and eye strain 133M presbyopes3 Phase 3 ONGOING: Dim light disturbances in keratorefractive
patients 600-700K laser vision correction procedures per year4; 35% of LASIK patients report dim light disturbances5 ^$10M milestone payment received upon approval of the first indication for Phentolamine Ophthalmic Solution 0.75%;
1. Ryzumvi. Approved for reversal of pharmacologically-induced mydriasis produced by adrenergic agonists (e.g., phenylephrine) or parasympatholytic agents (e.g., tropicamide) Prescribing Information. Ocuphire Pharma, Inc.; 2023. 2. Wilson FA,
et al. J Ophthalmol. 2015;2015:435606. 3. Berdahl J, et al. Clin Ophthalmol. 2020;14:3439-3450. Phase 3 trial being conducted in keratorefractive patients with visual disturbances under mesopic, low-contrast conditions; 4. Lindstrom RL.
Ocular Surgery News. April 1, 2019. Accessed February 9, 2025. https://www.healio.com/news/ophthalmology/20190329/millennials-will-be-the-next-target-for-laser-vision-correction 5. Mamalis N. J Cataract Refract Surg. 2014;40:343-344. LASIK,
laser assisted in situ keratomileusis. 26 Global Partnership for Phentolamine Ophthalmic Solution 0.75% Future double-digit royalty stream & potential development/commercial milestones up to $130M^ All Three Indications Have Sizable
U.S. Patient Populations
Differentiated MOA of Phentolamine is Designed to be Well-Suited for Presbyopia
and Decreased Visual Acuity Under Low Light Conditions Favorable tolerability profile, with no reported incidence of retinal tears or retinal detachment, and minimal to no headaches or dimming Fast onset of action and extended durability,
with reduction of pupil size lasting over 20 hours Once-daily evening dosing enables improved near vision immediately upon awakening MOA, mechanism of action. STUDIES TO DATE HAVE SHOWN: 27 Our Objective Provide a safe, long-lasting and
effective solution that restores near vision and enhances overall visual performance in daylight and low-light conditions
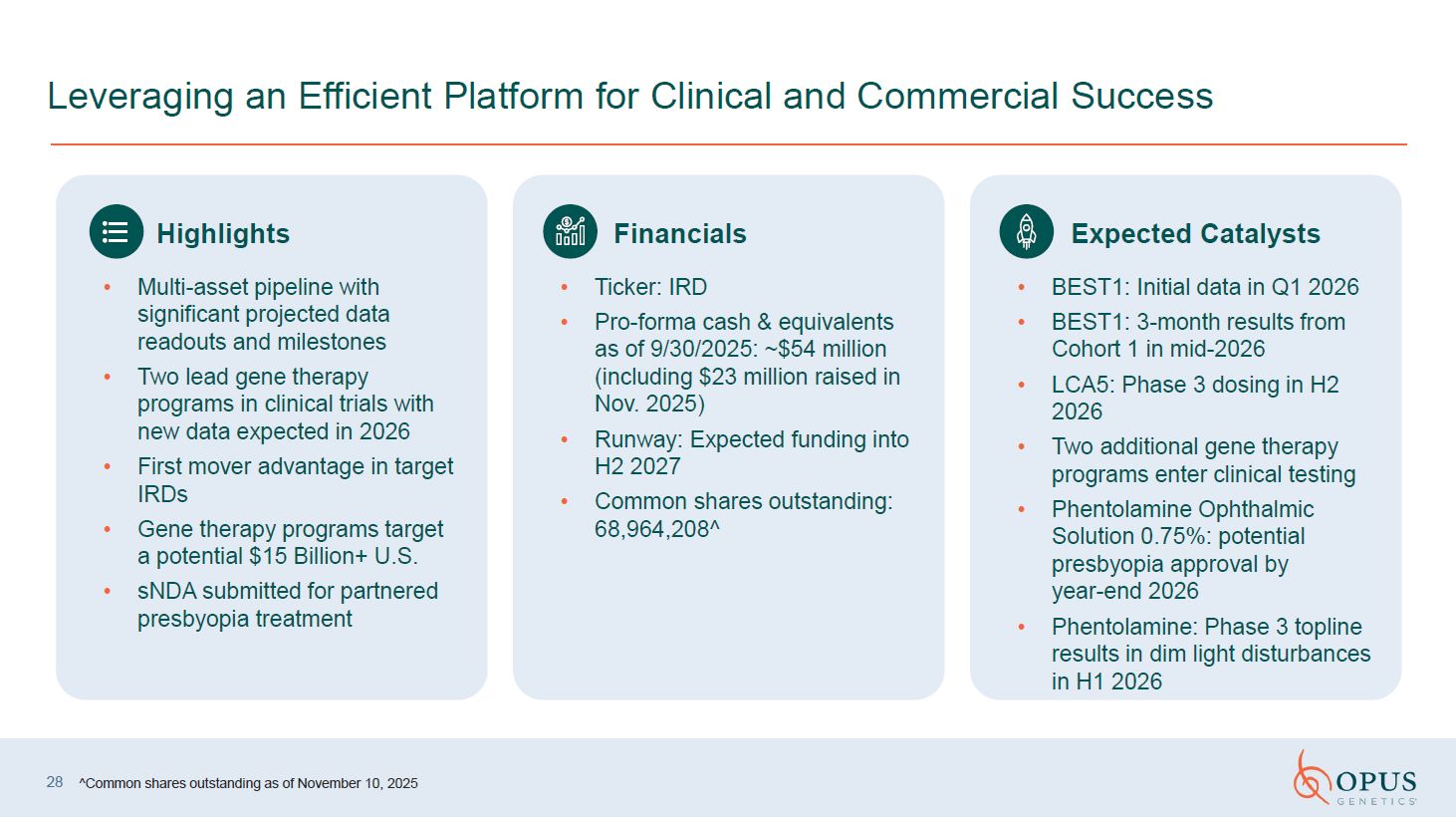
Leveraging an Efficient Platform for Clinical and Commercial Success ^Common
shares outstanding as of November 10, 2025 28 Highlights Multi-asset pipeline with significant projected data readouts and milestones Two lead gene therapy programs in clinical trials with new data expected in 2026 First mover
advantage in target IRDs Gene therapy programs target a potential $15 Billion+ U.S. sNDA submitted for partnered presbyopia treatment Financials Ticker: IRD Pro-forma cash & equivalents as of 9/30/2025: ~$54 million (including $23
million raised in Nov. 2025) Runway: Expected funding into H2 2027 Common shares outstanding: 68,964,208^ Expected Catalysts BEST1: Initial data in Q1 2026 BEST1: 3-month results from Cohort 1 in mid-2026 LCA5: Phase 3 dosing in H2
2026 Two additional gene therapy programs enter clinical testing Phentolamine Ophthalmic Solution 0.75%: potential presbyopia approval by year-end 2026 Phentolamine: Phase 3 topline results in dim light disturbances in H1 2026

“Impossible is a dare to science.” – Will Reeve, Good Morning America
feature story on Opus LCA5 patient Nasdaq: IRD