EXHIBIT 99.2
Published on November 15, 2021
Exhibit 99.2

Restore Vision & Clarity Ocuphire Corporate Presentation November 15, 2021 Mina Sooch
CEO Exhibit 99.2

2 Disclosures and Forward Looking Statements This presentation contains “forward-looking statements”
within the meaning of the Private Securities Litigation Reform Act of 1995. Such statements include, but are not limited to, statements concerning Ocuphire Pharma, Inc.’s (“Ocuphire” or the “Company”) product candidates and future milestones,
including the potential for Nyxol to be a “best in class” presbyopia drop. These forward-looking statements are based upon the Company’s current expectations and involve assumptions that may never materialize or may prove to be incorrect.
Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, including, without limitation: (i) timing or ability for the company to
achieve its targeted milestones; (ii) the success and timing of regulatory submissions and pre-clinical and clinical trials; (iii) regulatory requirements or developments; (iv) changes to clinical trial designs and regulatory pathways; (v)
changes in capital resource requirements; (vi) risks related to the inability of the Company to obtain sufficient additional capital to continue to advance its product candidates and its preclinical programs; (vii) legislative, regulatory,
political and economic developments, and (viii) the effects of COVID-19 on clinical programs and business operations. The foregoing review of important factors that could cause actual events to differ from expectations should not be construed
as exhaustive and should be read in conjunction with statements that are included herein and elsewhere, including the risk factors detailed in documents that have been and may be filed by the Company from time to time with the SEC. All
forward-looking statements contained in this presentation speak only as of the date on which they were made. The Company undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date
on which they were made.The Company makes no representation or warranty, express or implied, as to the accuracy or completeness of the information contained in or incorporated by reference into this presentation. Nothing contained in or
incorporated by reference into this presentation is, or shall be relied upon as, a promise or representation by the Company as to the past or future. The Company assumes no responsibility for the accuracy or completeness of any such
information. This presentation may not be reproduced or provided to any other person (other than your advisor) without our prior written consent. By accepting delivery of this presentation, you agree to the foregoing and agree to return this
presentation and any documents related thereto and any copies thereof to us or to destroy the same if you do not make an investment in any securities. The information contain within this presentation shall not, except as hereinafter provided,
without the prior written consent of the Company, be disclosed by you or your representatives in any manner whatsoever, in whole or in part, and shall not be used by you or your representatives other than for the purpose of evaluating the
transaction described herein. By accepting delivery of this presentation you further acknowledge and agree aware of the restrictions imposed by the United States securities laws on the purchase or sale of securities by any person who has
received material, nonpublic information from the issuer of the securities or any affiliate thereof and on the communication of such information to any other person when it is reasonably foreseeable that such other person is likely to purchase
or sell such securities in reliance on such information for so long as the information remains material and non- public. This presentation also contains estimates and other statistical data made by independent parties and by us relating to
market shares and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. The trademarks included herein are the property of the owners thereof
and are used for reference purposes only. Such use should not be construed as an endorsement of such products. November 6, 2020
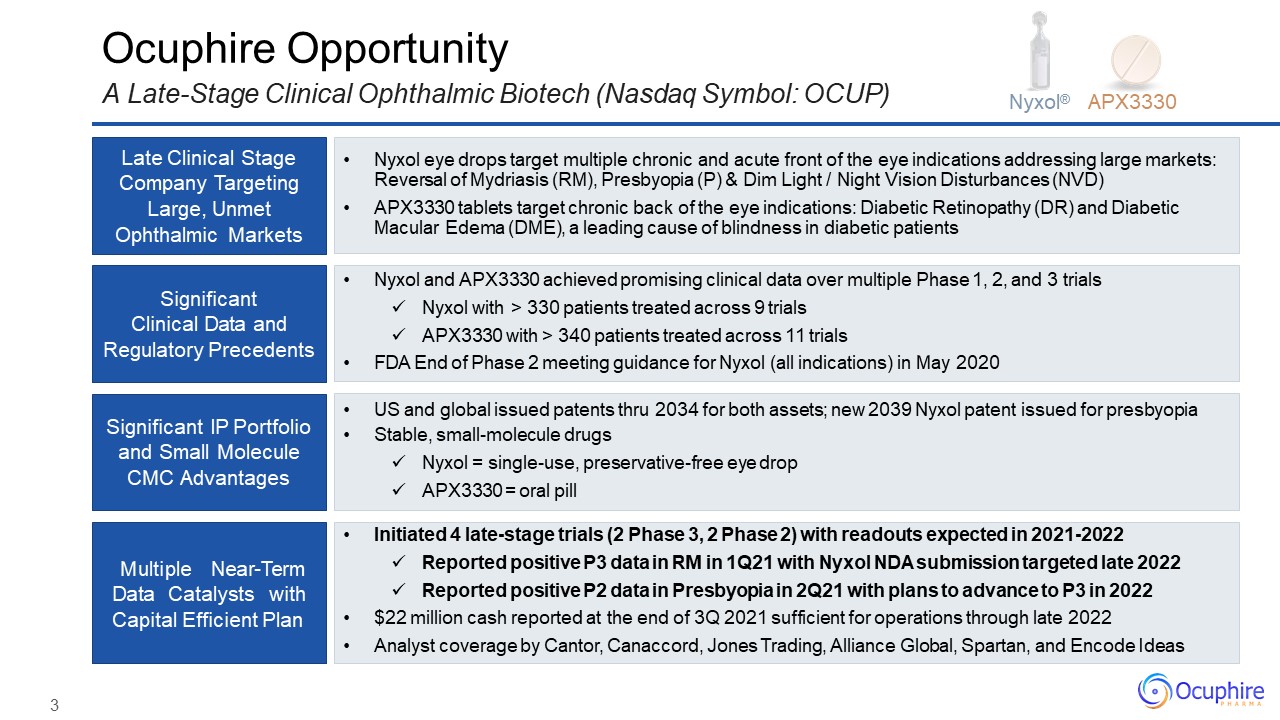
3 Late Clinical Stage Company Targeting Large, Unmet Ophthalmic Markets Significant Clinical Data
andRegulatory Precedents Significant IP Portfolio and Small Molecule CMC Advantages Multiple Near-Term Data Catalysts with Capital Efficient Plan Nyxol eye drops target multiple chronic and acute front of the eye indications addressing large
markets: Reversal of Mydriasis (RM), Presbyopia (P) & Dim Light / Night Vision Disturbances (NVD)APX3330 tablets target chronic back of the eye indications: Diabetic Retinopathy (DR) and Diabetic Macular Edema (DME), a leading cause of
blindness in diabetic patients Nyxol and APX3330 achieved promising clinical data over multiple Phase 1, 2, and 3 trialsNyxol with > 330 patients treated across 9 trialsAPX3330 with > 340 patients treated across 11 trialsFDA End of Phase
2 meeting guidance for Nyxol (all indications) in May 2020 US and global issued patents thru 2034 for both assets; new 2039 Nyxol patent issued for presbyopiaStable, small-molecule drugsNyxol = single-use, preservative-free eye dropAPX3330 =
oral pill Initiated 4 late-stage trials (2 Phase 3, 2 Phase 2) with readouts expected in 2021-2022Reported positive P3 data in RM in 1Q21 with Nyxol NDA submission targeted late 2022Reported positive P2 data in Presbyopia in 2Q21 with plans to
advance to P3 in 2022$22 million cash reported at the end of 3Q 2021 sufficient for operations through late 2022Analyst coverage by Cantor, Canaccord, Jones Trading, Alliance Global, Spartan, and Encode Ideas Ocuphire OpportunityA Late-Stage
Clinical Ophthalmic Biotech (Nasdaq Symbol: OCUP) Nyxol® APX3330

4 Ocuphire Management TeamDecades of Biotech and Drug Development Experience Mina Sooch,
MBA President & CEO and Founder Drey Coleman VP, Clinical Operations Amy Rabourn, CPA VP, Finance Charlie Hoffmann, MBA VP Corporate Developmentand Operations Mitch Brigell, PhDHead, Clinical
Developmentand Strategy Daniela Oniciu, PhD Global Head, R&D, Chemistry and Product Development Ronil Patel, MSSenior Director BD andMarket Strategy Chris Ernst Global Head, QA and Manufacturing Barbara Withers, PhDVP,
Clinical and Regulatory Strategy
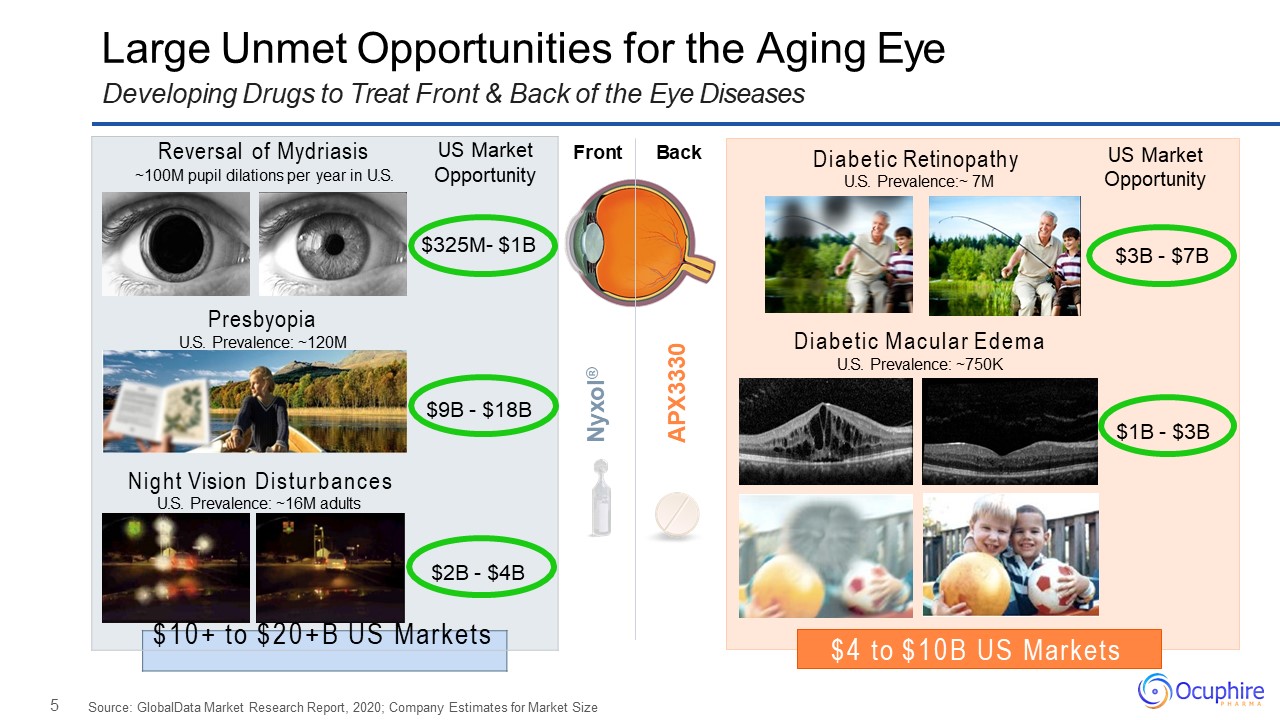
5 Large Unmet Opportunities for the Aging EyeDeveloping Drugs to Treat Front & Back of the
Eye Diseases Source: GlobalData Market Research Report, 2020; Company Estimates for Market Size Front Back Nyxol® APX3330 Reversal of Mydriasis US Market~100M pupil dilations per year in U.S. Opportunity$325M- $1BPresbyopiaU.S.
Prevalence: ~120M$9B - $18BNight Vision DisturbancesU.S. Prevalence: ~16M adults$2B - $4B $10+ to $20+B US Markets $4 to $10B US Markets Diabetic RetinopathyU.S. Prevalence:~ 7M US Market Opportunity $3B - $7BDiabetic Macular
EdemaU.S. Prevalence: ~750K$1B - $3B
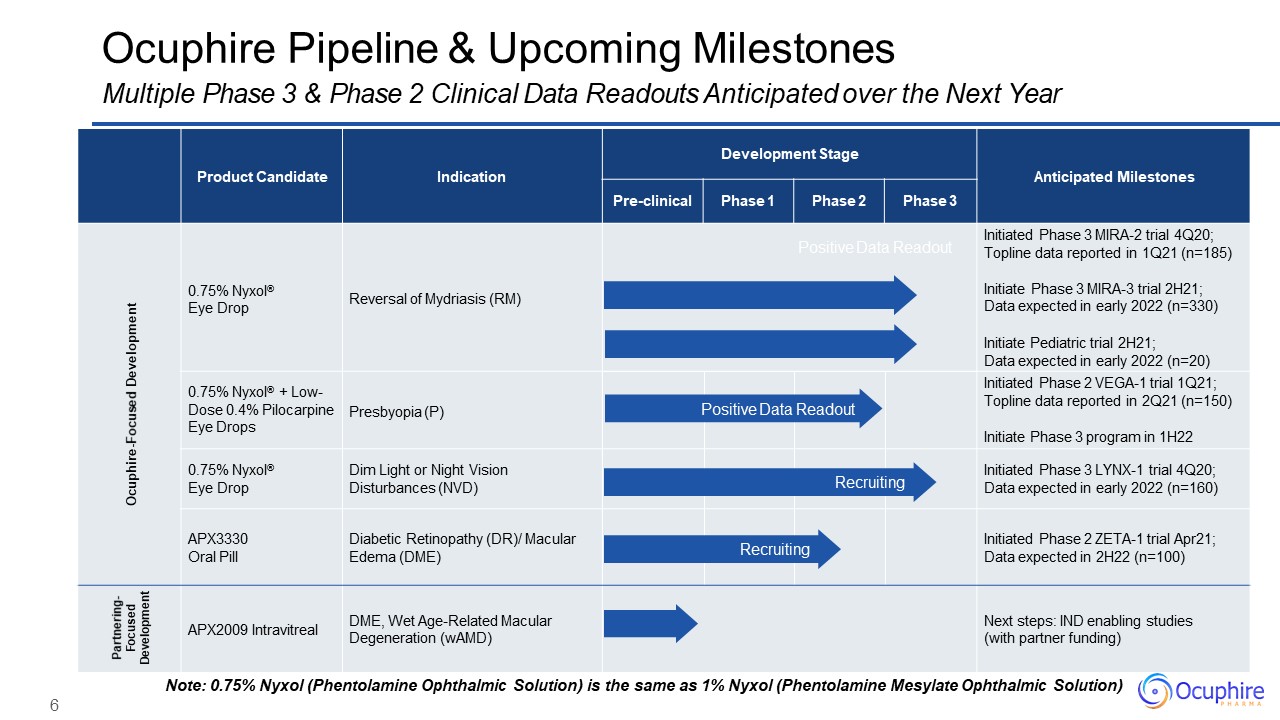
6 Product Candidate Indication Development Stage Anticipated
Milestones Pre-clinical Phase 1 Phase 2 Phase 3 Ocuphire-Focused Development 0.75% Nyxol®Eye Drop Reversal of Mydriasis (RM) Positive Data Readout Initiated Phase 3 MIRA-2 trial 4Q20; Topline data reported in 1Q21
(n=185)Initiate Phase 3 MIRA-3 trial 2H21; Data expected in early 2022 (n=330)Initiate Pediatric trial 2H21;Data expected in early 2022 (n=20) 0.75% Nyxol® + Low- Dose 0.4% Pilocarpine Eye Drops Presbyopia (P) Positive Data
Readout Initiated Phase 2 VEGA-1 trial 1Q21; Topline data reported in 2Q21 (n=150)Initiate Phase 3 program in 1H22 0.75% Nyxol®Eye Drop Dim Light or Night Vision Disturbances (NVD) Recruiting Initiated Phase 3 LYNX-1 trial
4Q20; Data expected in early 2022 (n=160) APX3330Oral Pill Diabetic Retinopathy (DR)/ Macular Edema (DME) Recruiting Initiated Phase 2 ZETA-1 trial Apr21; Data expected in 2H22 (n=100) Partnering- Focused Development APX2009
Intravitreal DME, Wet Age-Related Macular Degeneration (wAMD) Next steps: IND enabling studies (with partner funding) Ocuphire Pipeline & Upcoming MilestonesMultiple Phase 3 & Phase 2 Clinical Data Readouts Anticipated over
the Next Year Note: 0.75% Nyxol (Phentolamine Ophthalmic Solution) is the same as 1% Nyxol (Phentolamine Mesylate Ophthalmic Solution)
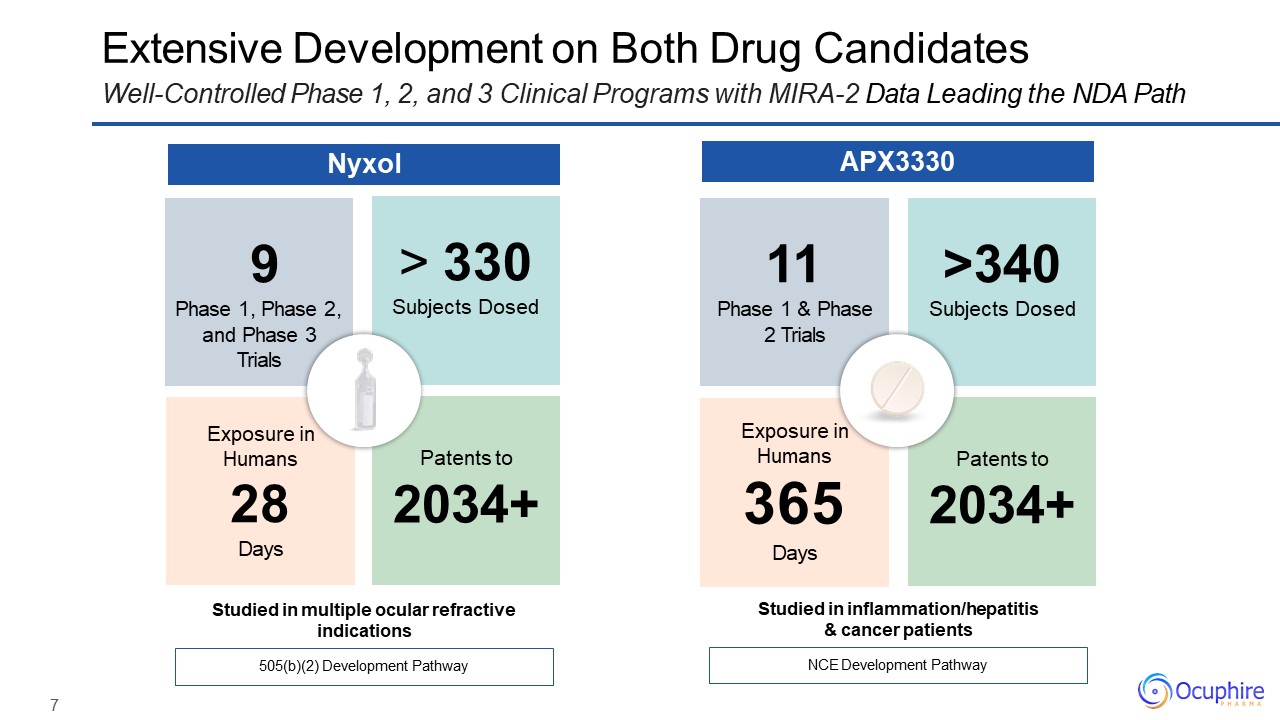
7 11Phase 1 & Phase 2 Trials >340Subjects Dosed Exposure in Humans365Days Patents
to2034+ Extensive Development on Both Drug CandidatesWell-Controlled Phase 1, 2, and 3 Clinical Programs with MIRA-2 Data Leading the NDA Path NCE Development Pathway Studied in inflammation/hepatitis & cancer patients 9Phase 1,
Phase 2,and Phase 3 Trials 330Subjects Dosed Exposure in Humans28Days Patents to2034+ 505(b)(2) Development Pathway Studied in multiple ocular refractive indications Nyxol APX3330
8 Nyxol® Phentolamine Mesylate Presbyopia RM Reversal of Mydriasis NVD Night Vision
Disturbances P
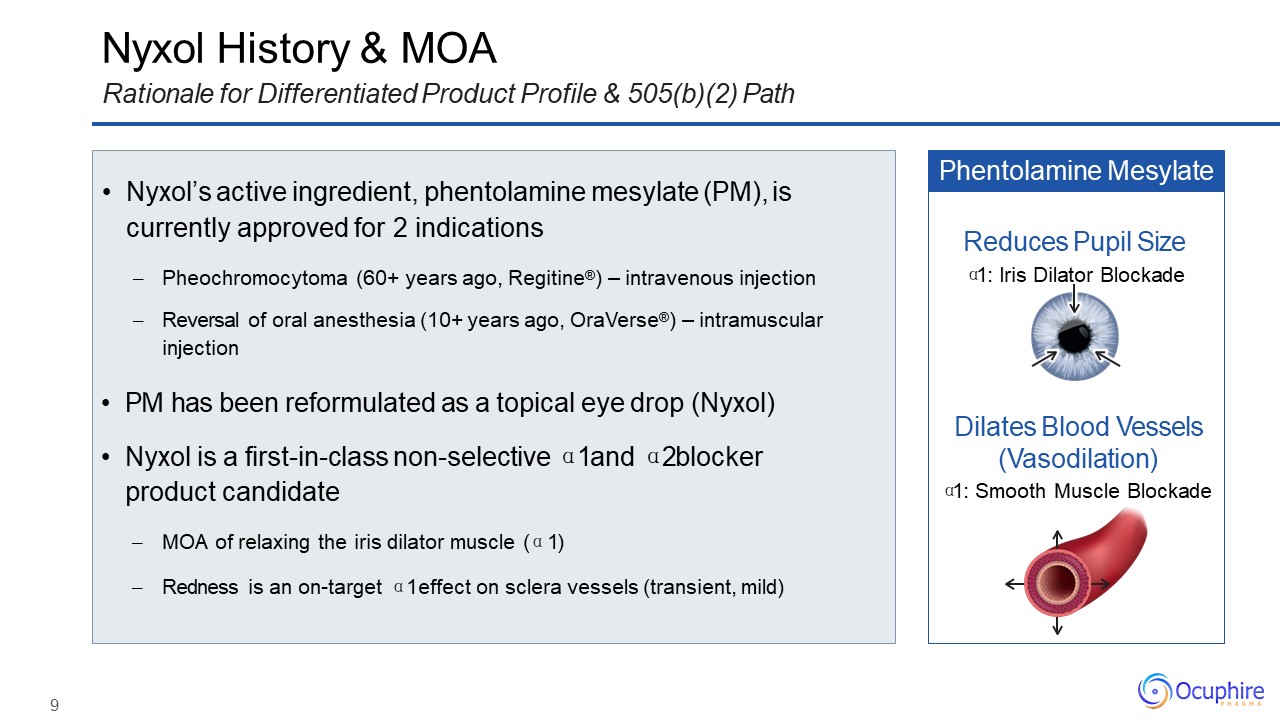
9 Nyxol History & MOARationale for Differentiated Product Profile & 505(b)(2) Path Nyxol’s
active ingredient, phentolamine mesylate (PM), is currently approved for 2 indicationsPheochromocytoma (60+ years ago, Regitine®) – intravenous injectionReversal of oral anesthesia (10+ years ago, OraVerse®) – intramuscular injection PM has
been reformulated as a topical eye drop (Nyxol)Nyxol is a first-in-class non-selective α1 and α2 blocker product candidateMOA of relaxing the iris dilator muscle (α1)Redness is an on-target α1 effect on sclera vessels (transient,
mild) Phentolamine Mesylate Dilates Blood Vessels (Vasodilation)α1: Smooth Muscle Blockade Reduces Pupil Sizeα1: Iris Dilator Blockade
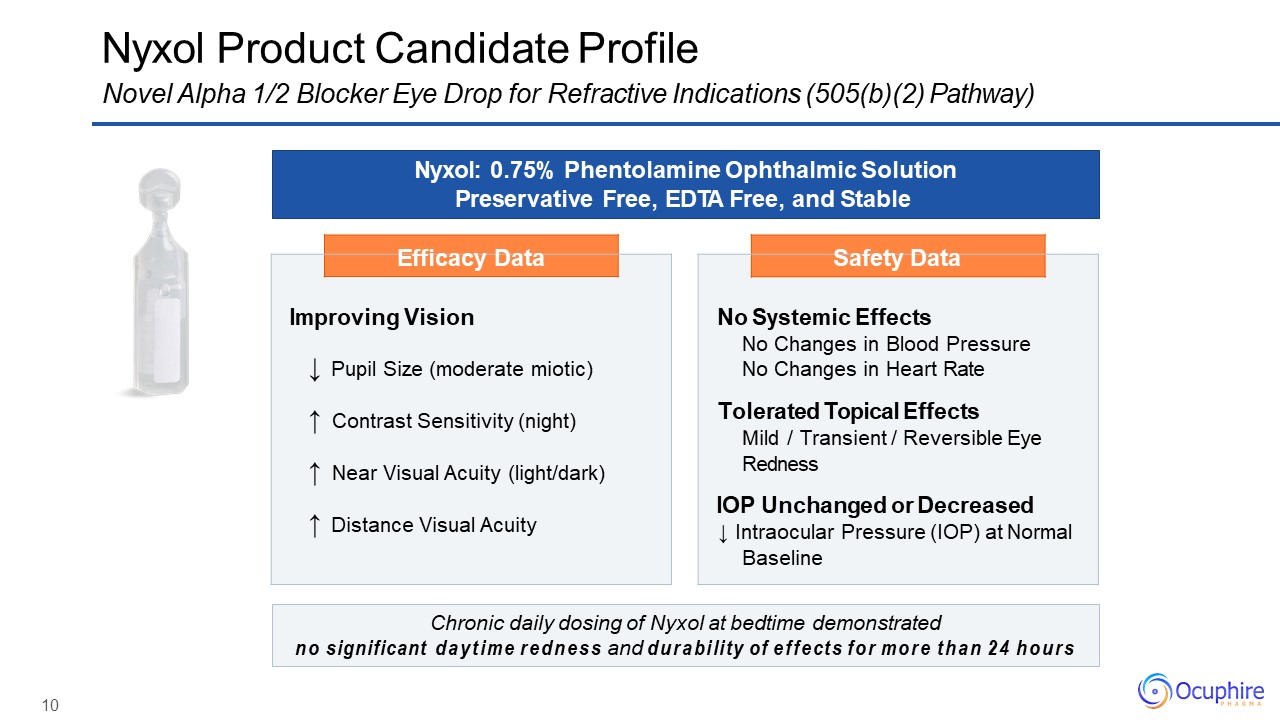
10 Efficacy Data Improving Vision↓ Pupil Size (moderate miotic)↑ Contrast Sensitivity
(night)↑ Near Visual Acuity (light/dark)↑ Distance Visual Acuity Safety Data No Systemic EffectsNo Changes in Blood Pressure No Changes in Heart RateTolerated Topical EffectsMild / Transient / Reversible Eye RednessIOP Unchanged
or Decreased↓ Intraocular Pressure (IOP) at Normal Baseline Nyxol Product Candidate ProfileNovel Alpha 1/2 Blocker Eye Drop for Refractive Indications (505(b)(2) Pathway) Nyxol: 0.75% Phentolamine Ophthalmic Solution Preservative Free,
EDTA Free, and Stable Chronic daily dosing of Nyxol at bedtime demonstratedno significant daytime redness and durability of effects for more than 24 hours
11 Nyxol® Phentolamine Mesylate NVD P Presbyopia RM Reversal of Mydriasis Night
Vision Disturbances 0.4% +
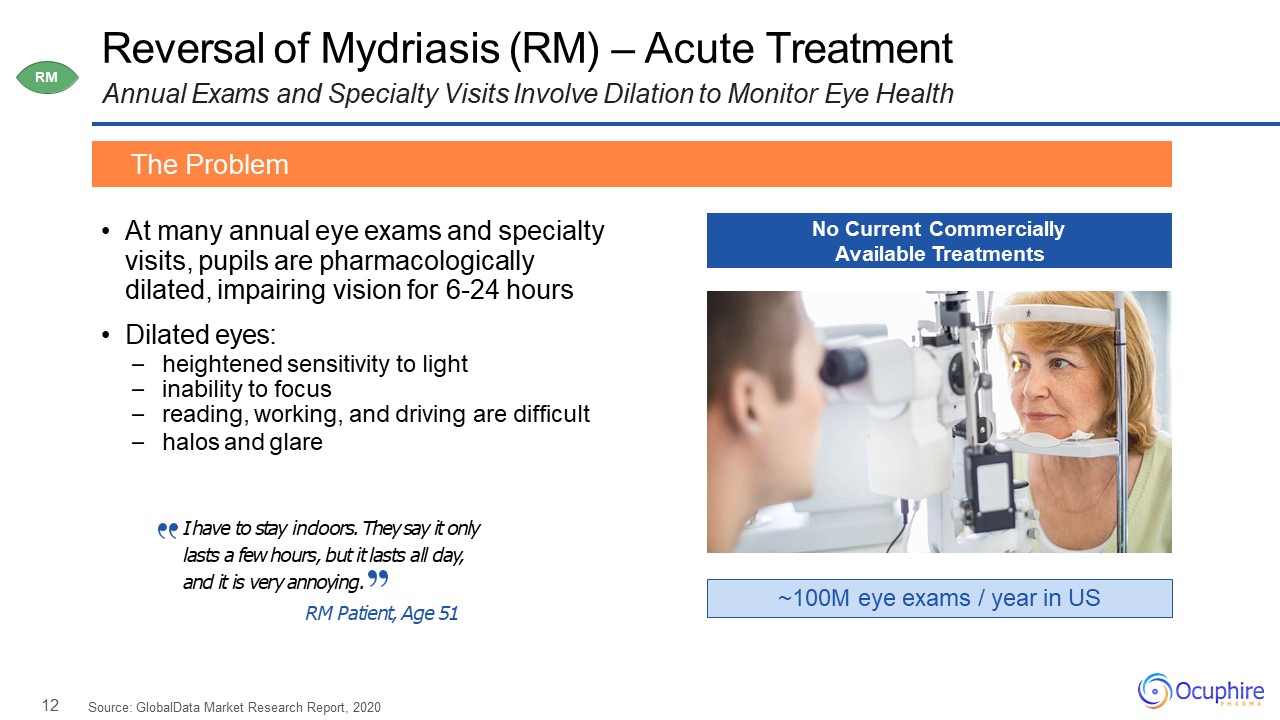
12 Reversal of Mydriasis (RM) – Acute TreatmentAnnual Exams and Specialty Visits Involve Dilation to
Monitor Eye Health At many annual eye exams and specialty visits, pupils are pharmacologically dilated, impairing vision for 6-24 hoursDilated eyes:heightened sensitivity to lightinability to focusreading, working, and driving are
difficulthalos and glare The Problem ~100M eye exams / year in US No Current Commercially Available Treatments RM Source: GlobalData Market Research Report, 2020 I have to stay indoors. They say it only lasts a few hours, but it lasts
all day, and it is very annoying.RM Patient, Age 51
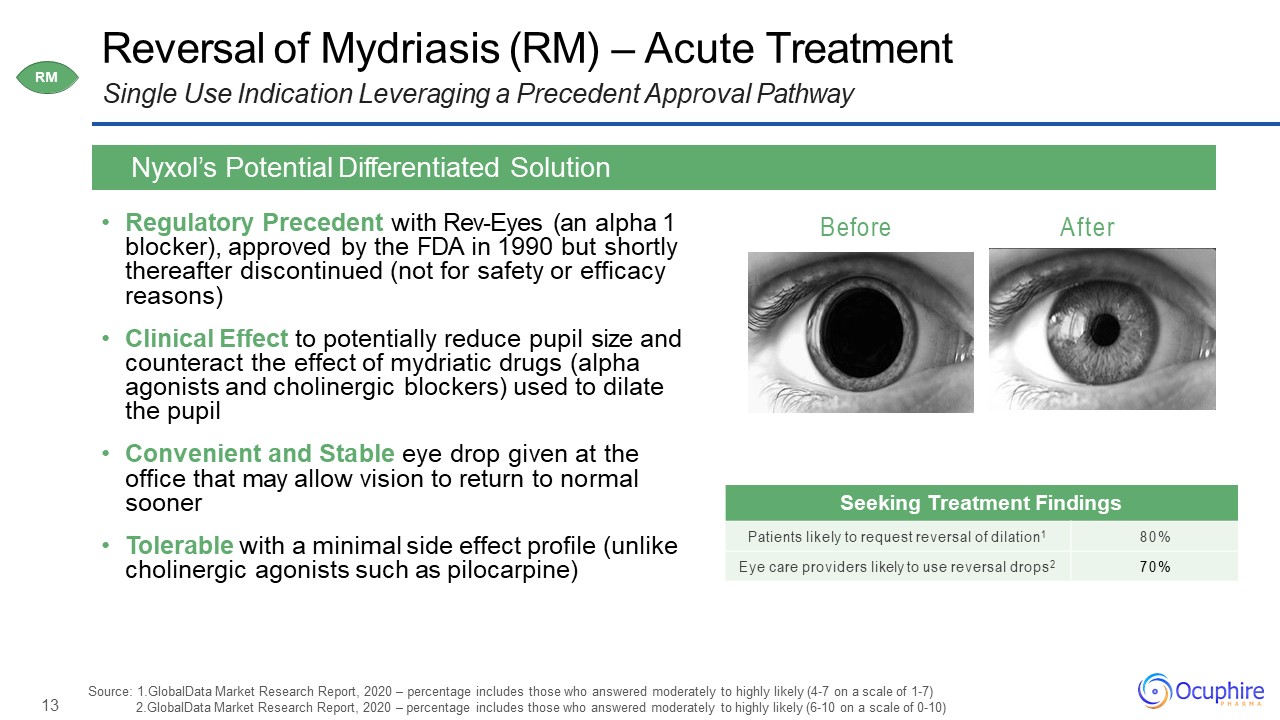
13 Before After Reversal of Mydriasis (RM) – Acute TreatmentSingle Use Indication Leveraging a
Precedent Approval Pathway Regulatory Precedent with Rev-Eyes (an alpha 1 blocker), approved by the FDA in 1990 but shortly thereafter discontinued (not for safety or efficacy reasons)Clinical Effect to potentially reduce pupil size and
counteract the effect of mydriatic drugs (alpha agonists and cholinergic blockers) used to dilate the pupilConvenient and Stable eye drop given at the office that may allow vision to return to normal soonerTolerable with a minimal side effect
profile (unlike cholinergic agonists such as pilocarpine) Nyxol’s Potential Differentiated Solution Seeking Treatment Findings Patients likely to request reversal of dilation1 80% Eye care providers likely to use reversal
drops2 70% Source: 1.GlobalData Market Research Report, 2020 – percentage includes those who answered moderately to highly likely (4-7 on a scale of 1-7) 2.GlobalData Market Research Report, 2020 – percentage includes those who answered
moderately to highly likely (6-10 on a scale of 0-10) RM
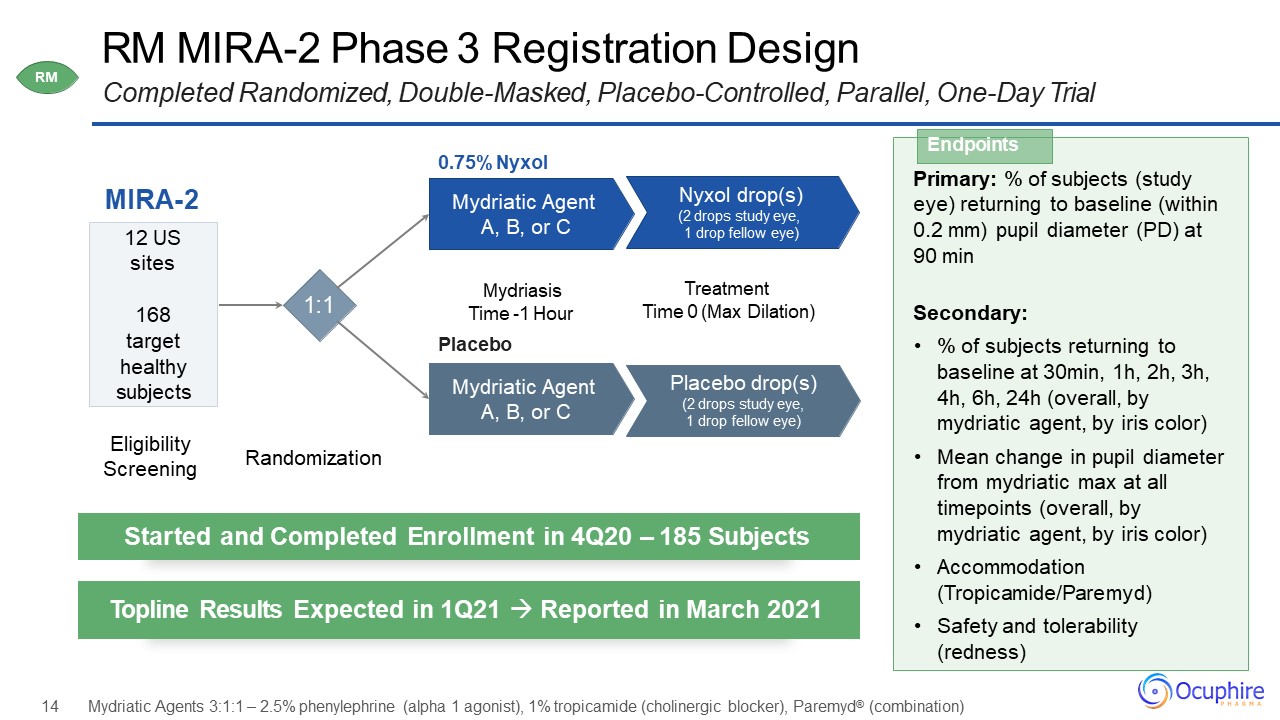
RM MIRA-2 Phase 3 Registration Design Completed Randomized, Double-Masked, Placebo-Controlled,
Parallel, One-Day Trial MIRA-2 0.75% NyxolMydriatic Agent A, B, or C 12 USsites168target healthy subjects Nyxol drop(s)(2 drops study eye, 1 drop fellow eye) Mydriasis Time -1 HourPlaceboMydriatic Agent A, B, or C Placebo
drop(s)(2 drops study eye, 1 drop fellow eye) TreatmentTime 0 (Max Dilation) Primary: % of subjects (study eye) returning to baseline (within0.2 mm) pupil diameter (PD) at 90 min Secondary:% of subjects returning to baseline at 30min,
1h, 2h, 3h, 4h, 6h, 24h (overall, by mydriatic agent, by iris color)Mean change in pupil diameter from mydriatic max at all timepoints (overall, by mydriatic agent, by iris color)Accommodation (Tropicamide/Paremyd)Safety and tolerability
(redness) Endpoints Started and Completed Enrollment in 4Q20 – 185 Subjects Topline Results Expected in 1Q21 Reported in March 2021 Eligibility Screening Randomization 14 Mydriatic Agents 3:1:1 – 2.5% phenylephrine (alpha 1
agonist), 1% tropicamide (cholinergic blocker), Paremyd® (combination) 1:1 RM
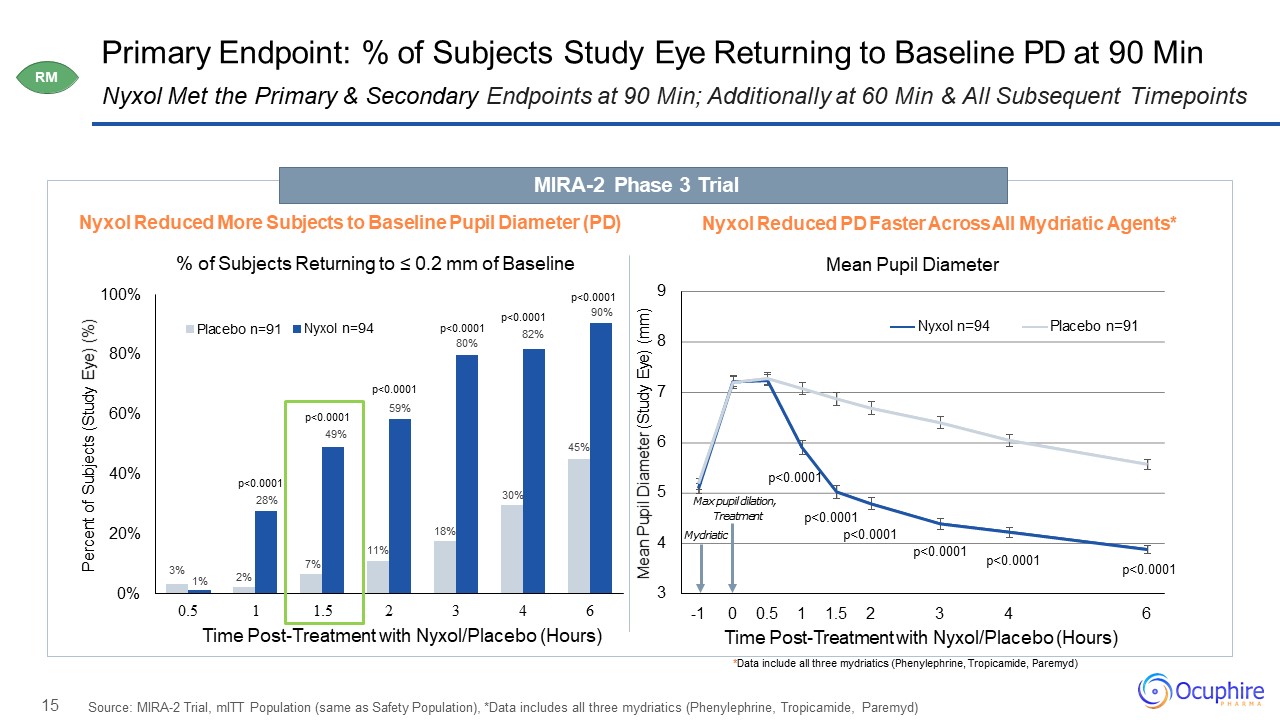
15 Primary Endpoint: % of Subjects Study Eye Returning to Baseline PD at 90 Min Source: MIRA-2 Trial,
mITT Population (same as Safety Population), *Data includes all three mydriatics (Phenylephrine, Tropicamide, Paremyd) Nyxol Met the Primary & Secondary Endpoints at 90 Min; Additionally at 60 Min & All Subsequent
Timepoints 2% 7% 11% 18% 30% 45% 3%1% 59% 0% 20% 40% 60% 80% 100% 0.5 Percent of Subjects (Study Eye) (%) 1 1.5 2 3 4 6Time Post-Treatment with Nyxol/Placebo (Hours) Placebo n=91 Nyxol
n=94 p<0.000190% p<0.000149% p<0.0001 p<0.000180% p<0.000182% p<0.000128% 3 4 5 6 7 8 9 6 Mean Pupil Diameter (Study Eye)
(mm) -1 0 0.5 1 1.5 2 3 4Time Post-Treatment with Nyxol/Placebo (Hours) Nyxol n=94 Placebo n=91 Max pupil dilation, Treatment Mydriatic p<0.0001 p<0.0001 p<0.0001 p<0.0001 Nyxol Reduced More Subjects to
Baseline Pupil Diameter (PD)% of Subjects Returning to ≤ 0.2 mm of Baseline Nyxol Reduced PD Faster Across All Mydriatic Agents*Mean Pupil Diameter MIRA-2 Phase 3 Trial *Data include all three mydriatics (Phenylephrine, Tropicamide,
Paremyd) p<0.0001p<0.0001 RM
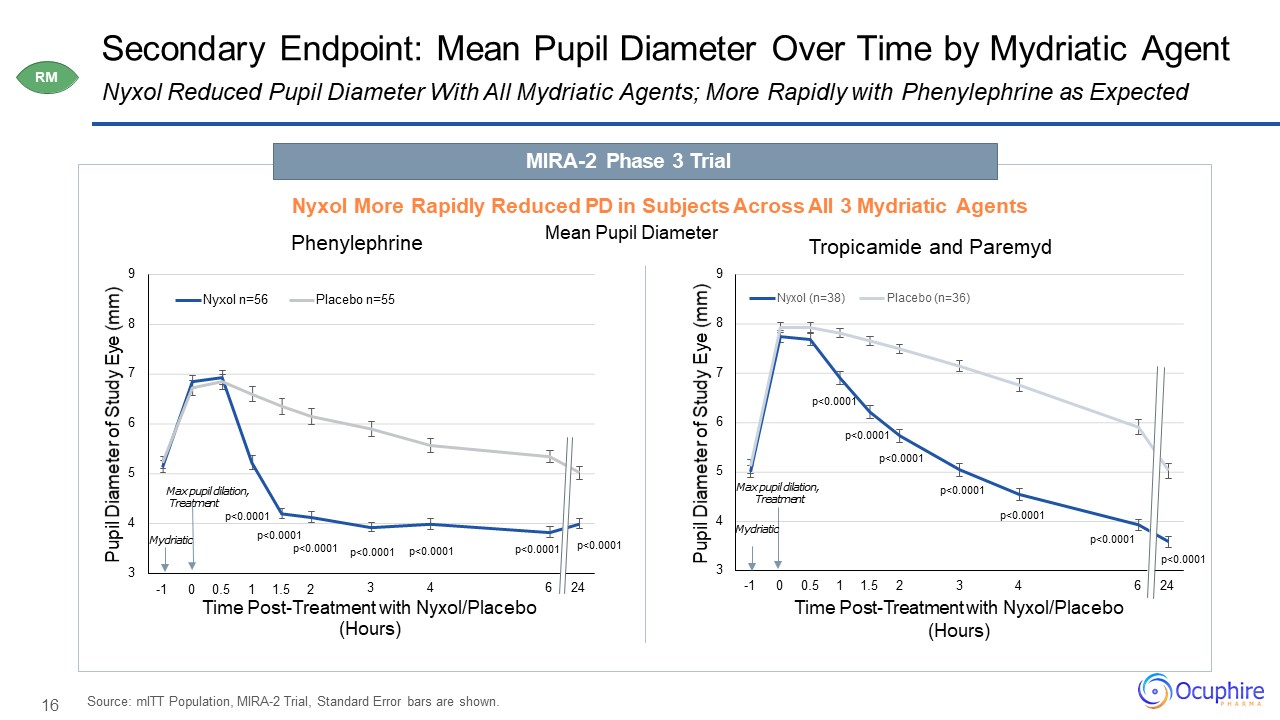
16 3 4 5 6 7 8 9 6 24 Pupil Diameter of Study
Eye (mm) -1 0 0.5 1 1.5 2 3 4Time Post-Treatment with Nyxol/Placebo (Hours) Tropicamide and Paremyd Nyxol (n=38) Placebo (n=36) 3 4 5 6 7 8 9 3 4 6 24 Pupil Diameter of Study Eye
(mm) Time Post-Treatment with Nyxol/Placebo (Hours) Phenylephrine Nyxol n=56 Placebo n=55 Secondary Endpoint: Mean Pupil Diameter Over Time by Mydriatic AgentNyxol Reduced Pupil Diameter With All Mydriatic Agents; More Rapidly with
Phenylephrine as Expected Source: mITT Population, MIRA-2 Trial, Standard Error bars are shown. Mydriatic Max pupil dilation, Treatment Max pupil dilation, Treatment Mydriatic MIRA-2 Phase 3 TrialNyxol More Rapidly
Reduced PD in Subjects Across All 3 Mydriatic Agents Mean Pupil Diameter RM p<0.0001 p<0.0001p<0.0001-1 0 0.5 1 1.5
2 p<0.0001 p<0.0001 p<0.0001 p<0.0001 p<0.0001 p<0.0001p<0.0001 p<0.0001 p<0.0001 p<0.0001 p<0.0001
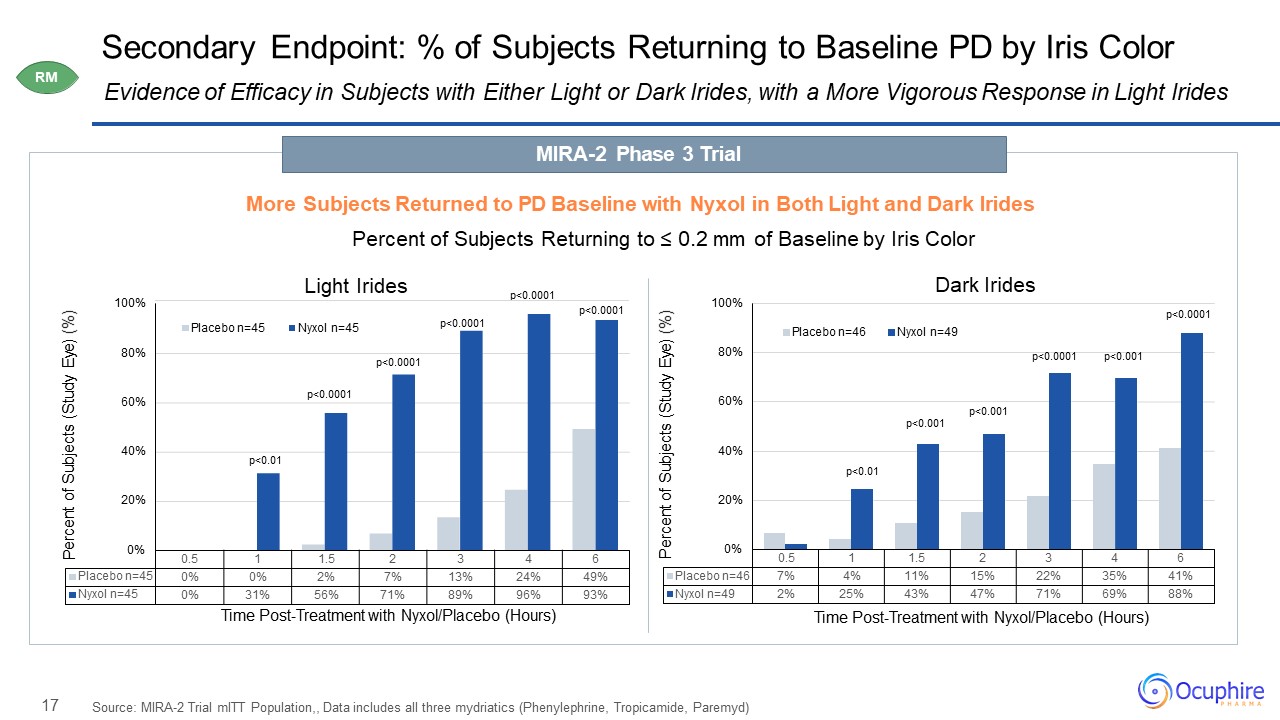
17 0.5 1 1.5 2 3 4 6 Placebo
n=46 7% 4% 11% 15% 22% 35% 41% Nyxol n=49 2% 25% 43% 47% 71% 69% 88% 0% 20% 40% 60% 80% 100% Percent of Subjects (Study Eye) (%) Time Post-Treatment with Nyxol/Placebo (Hours) Placebo n=46 Nyxol n=49 Secondary
Endpoint: % of Subjects Returning to Baseline PD by Iris Color Source: MIRA-2 Trial mITT Population,, Data includes all three mydriatics (Phenylephrine, Tropicamide, Paremyd) Evidence of Efficacy in Subjects with Either Light or Dark Irides,
with a More Vigorous Response in Light Irides 0% 20% 40% 60% 80% 100% P 0.5 1 1.5 2 3 4 6 Placebo n=45 0% 0% 2% 7% 13% 24% 49% Nyxol n=45 0% 31% 56% 71% 89% 96% 93% ercent of
Subjects (Study Eye) (%) Time Post-Treatment with Nyxol/Placebo (Hours) Placebo n=45 Nyxol n=45 p<0.0001 p<0.0001 p<0.01 p<0.0001
p<0.0001 p<0.0001 p<0.0001 p<0.001 p<0.001p<0.001 p<0.01 p<0.0001 MIRA-2 Phase 3 TrialMore Subjects Returned to PD Baseline with Nyxol in Both Light and Dark IridesPercent of Subjects Returning to ≤ 0.2 mm of
Baseline by Iris ColorLight Irides Dark Irides RM
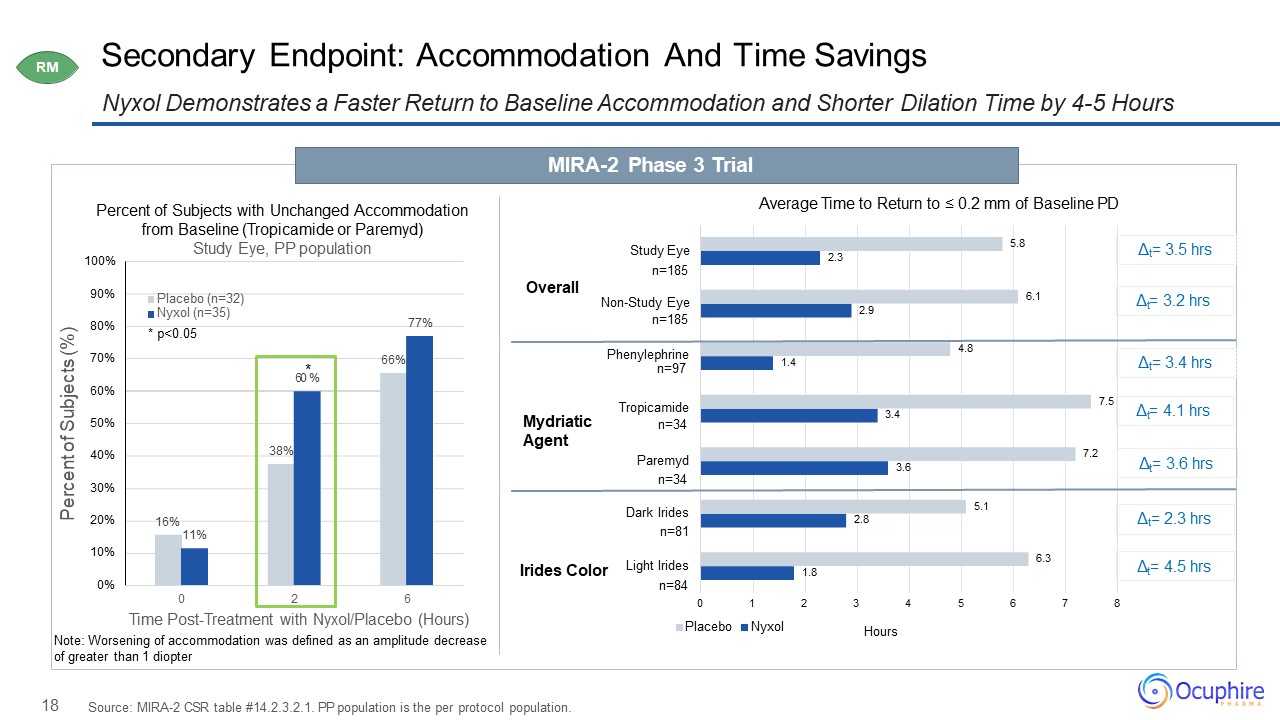
18 Secondary Endpoint: Accommodation And Time Savings Nyxol Demonstrates a Faster Return to Baseline
Accommodation and Shorter Dilation Time by 4-5 Hours Source: MIRA-2 CSR table #14.2.3.2.1. PP population is the per protocol population. Percent of Subjects with Unchanged Accommodation from Baseline (Tropicamide or Paremyd) Study Eye, PP
population MIRA-2 Phase 3 Trial RM 16% 38% 66% 11% 77% 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% 0 2 6 Percent of Subjects (%) Time Post-Treatment with Nyxol/Placebo (Hours)Note: Worsening of
accommodation was defined as an amplitude decrease of greater than 1 diopter Placebo (n=32) Nyxol (n=35) *
p<0.05 60*% 1.8 2.8 3.6 3.4 1.4 2.9 2.3 6.3 5.1 7.2 7.5 4.8 6.1 5.8 2 3 4 5 6 7 8 Hours 0 1Placebo Nyxol Overall Mydriatic Agent Irides Color Study Eyen=185 Non-Study
Eyen=185 Phenylephrinen=97 Tropicamiden=34 Dark Iridesn=81 Light Iridesn=84 Paremydn=34 Average Time to Return to ≤ 0.2 mm of Baseline PD Δt= 3.5 hrs t Δ = 3.2 hrs Δt= 3.4 hrs t Δ = 4.1 hrs t Δ = 3.6 hrs Δt= 2.3
hrs t Δ = 4.5 hrs
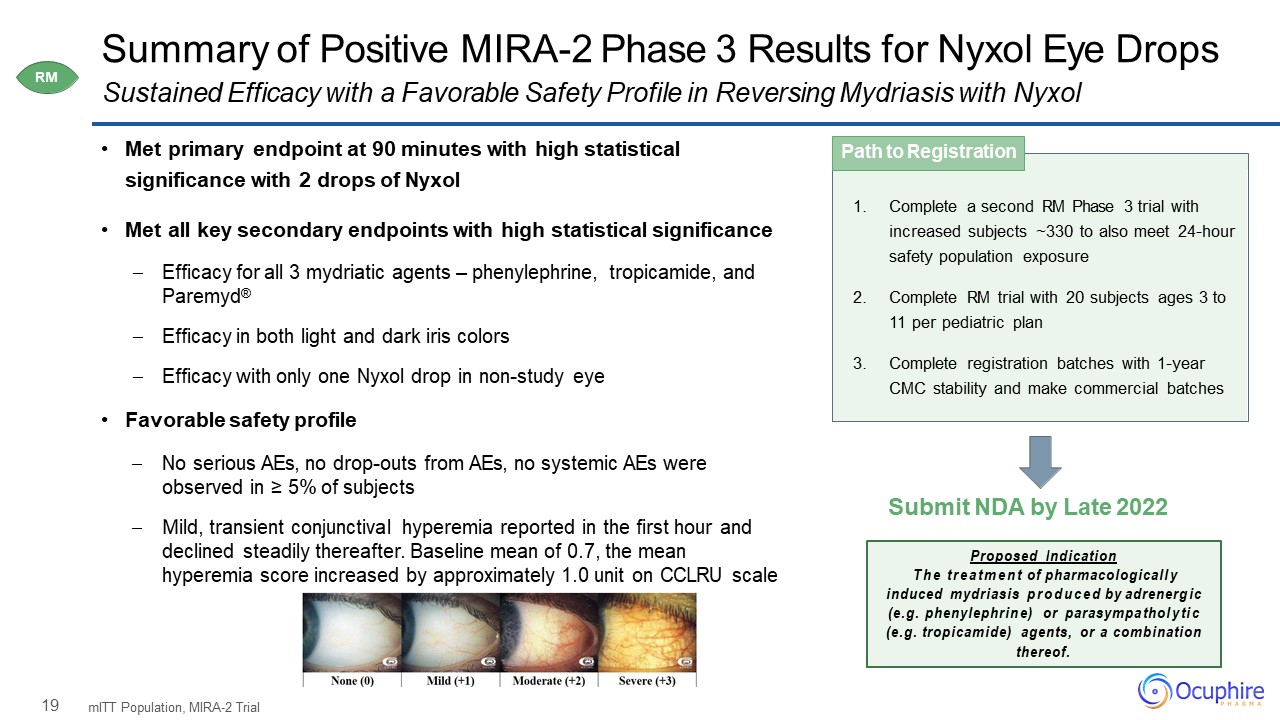
19 Summary of Positive MIRA-2 Phase 3 Results for Nyxol Eye Drops Met primary endpoint at 90
minutes with high statistical significance with 2 drops of NyxolMet all key secondary endpoints with high statistical significance Efficacy for all 3 mydriatic agents – phenylephrine, tropicamide, and Paremyd®Efficacy in both light and dark
iris colorsEfficacy with only one Nyxol drop in non-study eye Favorable safety profileNo serious AEs, no drop-outs from AEs, no systemic AEs were observed in ≥ 5% of subjectsMild, transient conjunctival hyperemia reported in the first hour and
declined steadily thereafter. Baseline mean of 0.7, the mean hyperemia score increased by approximately 1.0 unit on CCLRU scale mITT Population, MIRA-2 Trial Sustained Efficacy with a Favorable Safety Profile in Reversing Mydriasis with
Nyxol Complete a second RM Phase 3 trial with increased subjects ~330 to also meet 24-hour safety population exposureComplete RM trial with 20 subjects ages 3 to 11 per pediatric planComplete registration batches with 1-year CMC stability and
make commercial batches Path to Registration Submit NDA by Late 2022 Proposed IndicationThe treatment of pharmacologically induced mydriasis produced by adrenergic (e.g. phenylephrine) or parasympatholytic (e.g. tropicamide) agents,
or a combination thereof. RM
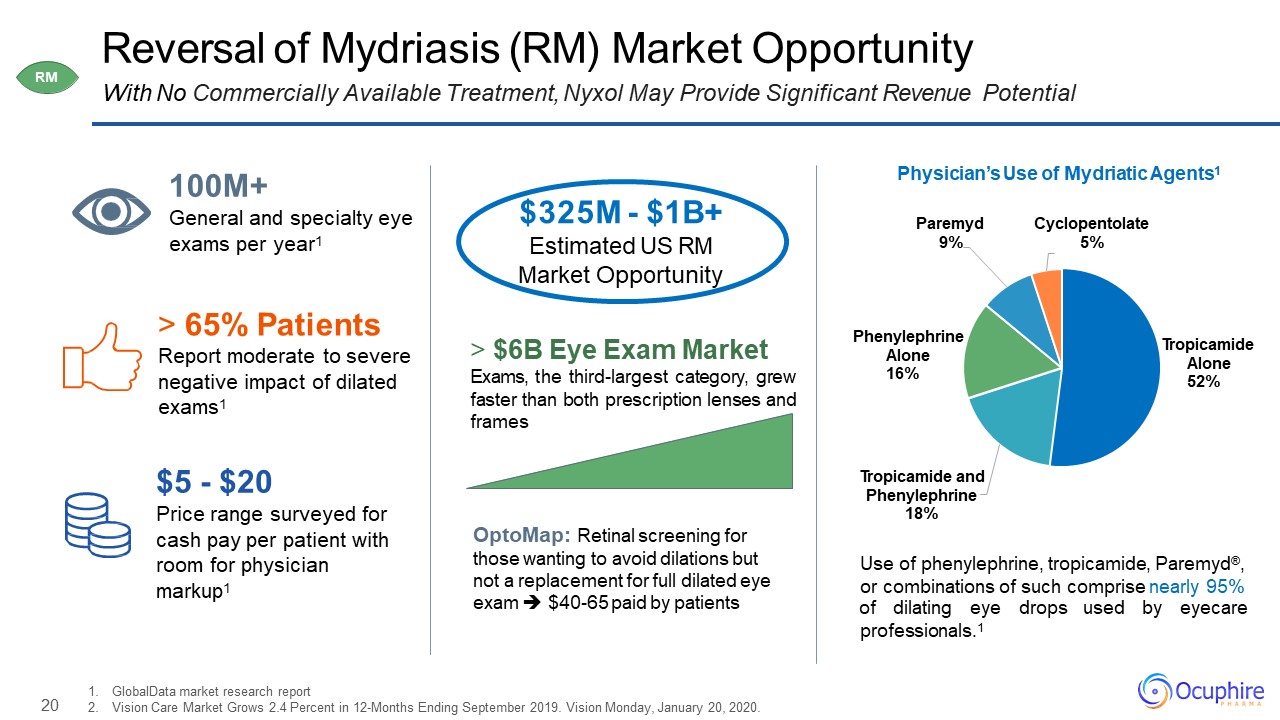
20 Reversal of Mydriasis (RM) Market OpportunityWith No Commercially Available Treatment, Nyxol May
Provide Significant Revenue Potential GlobalData market research reportVision Care Market Grows 2.4 Percent in 12-Months Ending September 2019. Vision Monday, January 20, 2020. 65% PatientsReport moderate to severe negative impact of dilated
exams1 $5 - $20Price range surveyed for cash pay per patient with room for physician markup1 $325M - $1B+Estimated US RM Market Opportunity 100M+General and specialty eye exams per year1 Tropicamide Alone
52% Tropicamide and Phenylephrine 18% Phenylephrine Alone 16% Paremyd 9% Cyclopentolate 5% Physician’s Use of Mydriatic Agents1 $6B Eye Exam MarketExams, the third-largest category, grew faster than both prescription lenses and
frames Use of phenylephrine, tropicamide, Paremyd®, or combinations of such comprise nearly 95% of dilating eye drops used by eyecare professionals.1 OptoMap: Retinal screening for those wanting to avoid dilations but not a replacement
for full dilated eye exam $40-65 paid by patients RM
21 Nyxol® Phentolamine Mesylate NVD P Presbyopia RM Night Vision
Disturbances Reversal of Mydriasis 0.4% +
22 2021: The Time for Presbyopia DropsHeadlines From Academia and Industry Articles Thru the Year with
an Early First Approval Sources: Academic review articles, journals, and publications “The correction of presbyopia remains ophthalmology’s ‘Holy Grail’…”-OIS 10/29/2021 P
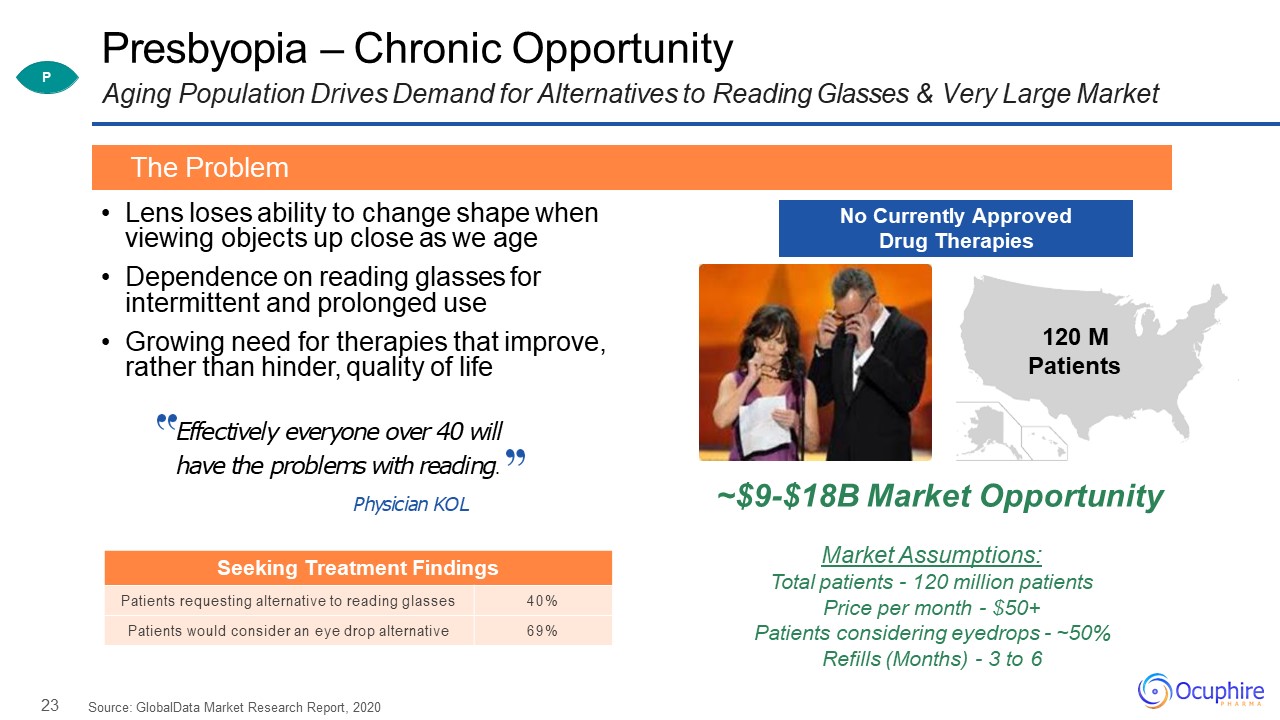
23 Presbyopia – Chronic OpportunityAging Population Drives Demand for Alternatives to Reading Glasses
& Very Large Market Lens loses ability to change shape when viewing objects up close as we ageDependence on reading glasses for intermittent and prolonged useGrowing need for therapies that improve, rather than hinder, quality of
life Source: GlobalData Market Research Report, 2020 The Problem P 120 MPatients No Currently Approved Drug Therapies Effectively everyone over 40 will have the problems with reading.Physician KOL Seeking Treatment
Findings Patients requesting alternative to reading glasses 40% Patients would consider an eye drop alternative 69% ~$9-$18B Market OpportunityMarket Assumptions:Total patients - 120 million patients Price per month - $50+Patients
considering eyedrops - ~50% Refills (Months) - 3 to 6
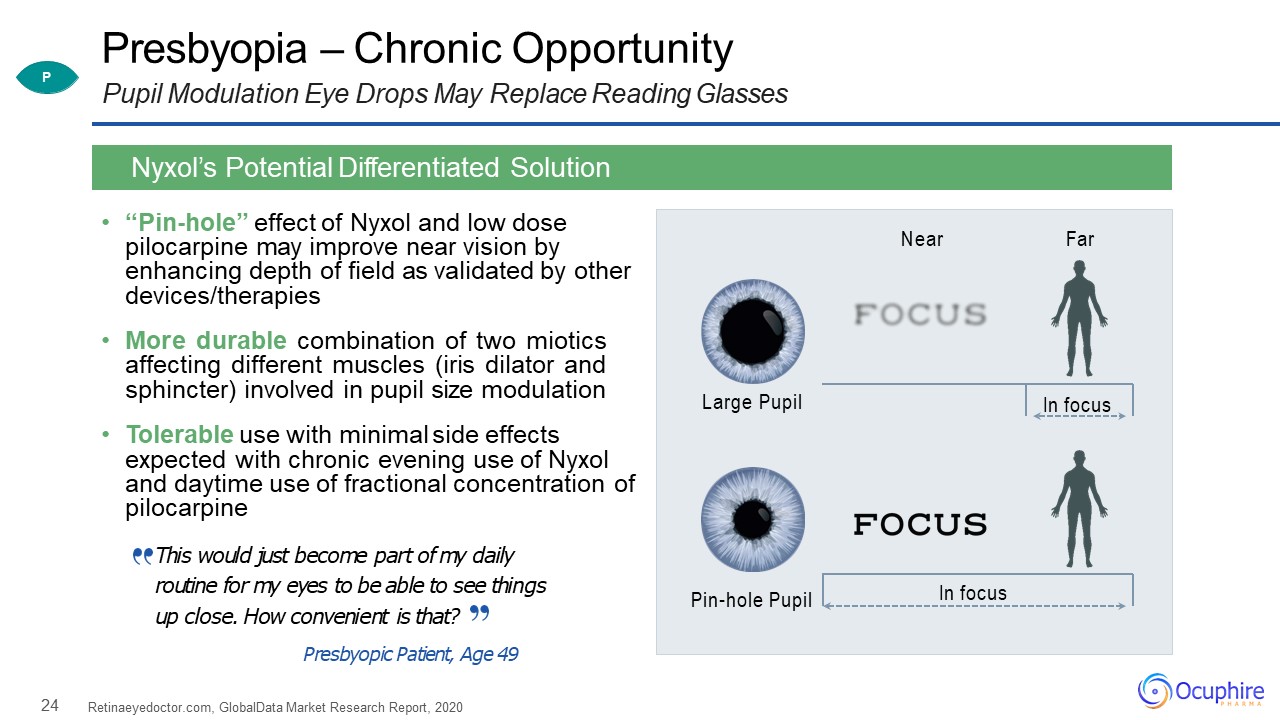
24 Presbyopia – Chronic OpportunityPupil Modulation Eye Drops May Replace Reading Glasses “Pin-hole”
effect of Nyxol and low dose pilocarpine may improve near vision by enhancing depth of field as validated by other devices/therapiesMore durable combination of two miotics affecting different muscles (iris dilator and sphincter) involved in
pupil size modulationTolerable use with minimal side effects expected with chronic evening use of Nyxol and daytime use of fractional concentration of pilocarpineThis would just become part of my daily routine for my eyes to be able to see
things up close. How convenient is that?Presbyopic Patient, Age 49 Retinaeyedoctor.com, GlobalData Market Research Report, 2020 Nyxol’s Potential Differentiated Solution Large Pupil Pin-hole Pupil Near Far In focus In
focus P
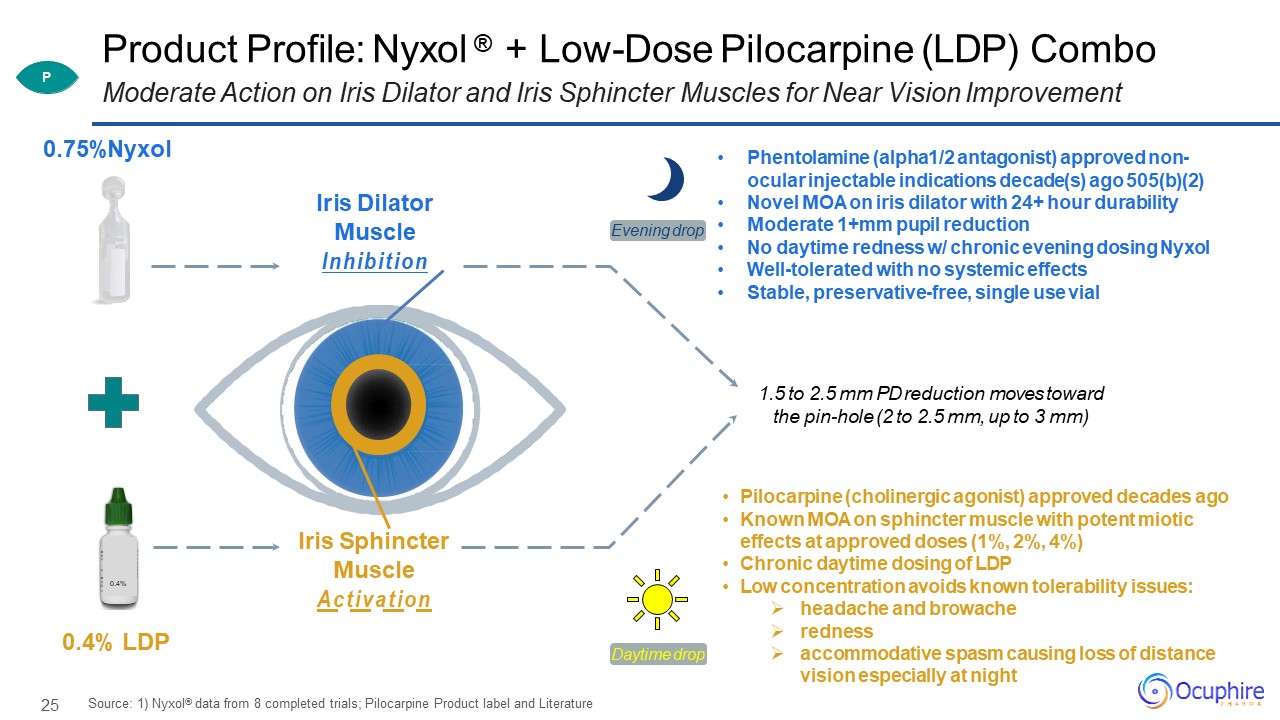
25 Product Profile: Nyxol ® + Low-Dose Pilocarpine (LDP) Combo Moderate Action on
Iris Dilator and Iris Sphincter Muscles for Near Vision Improvement 0.4% 0.75%Nyxol 0.4% LDP Iris Dilator Muscle Inhibition Iris Sphincter Muscle Activation Phentolamine (alpha1/2 antagonist) approved non- ocular injectable
indications decade(s) ago 505(b)(2)Novel MOA on iris dilator with 24+ hour durabilityModerate 1+mm pupil reductionNo daytime redness w/ chronic evening dosing NyxolWell-tolerated with no systemic effectsStable, preservative-free, single use
vial Pilocarpine (cholinergic agonist) approved decades agoKnown MOA on sphincter muscle with potent miotic effects at approved doses (1%, 2%, 4%)Chronic daytime dosing of LDPLow concentration avoids known tolerability issues:headache and
browacherednessaccommodative spasm causing loss of distance vision especially at night Source: 1) Nyxol® data from 8 completed trials; Pilocarpine Product label and Literature 1.5 to 2.5 mm PD reduction moves toward the pin-hole (2 to 2.5 mm,
up to 3 mm) Daytime drop Evening drop P
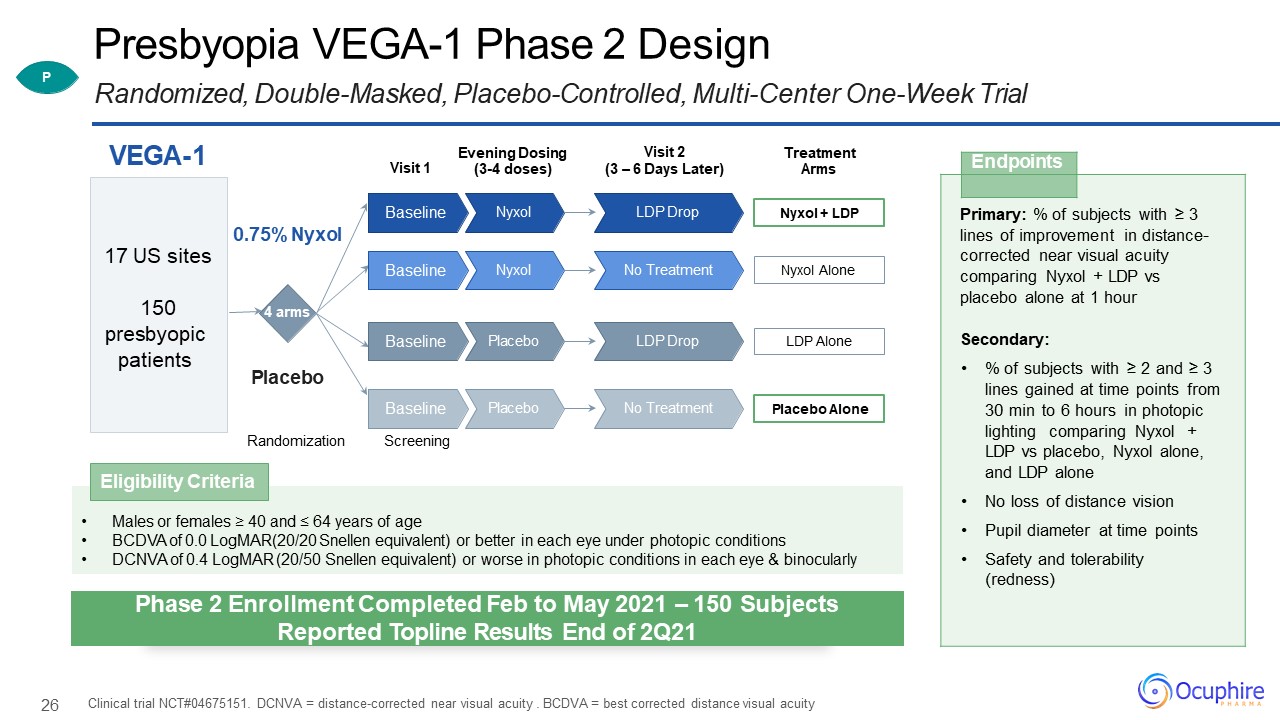
26 Presbyopia VEGA-1 Phase 2 DesignRandomized, Double-Masked, Placebo-Controlled, Multi-Center One-Week
Trial Clinical trial NCT#04675151. DCNVA = distance-corrected near visual acuity . BCDVA = best corrected distance visual acuity Endpoints Primary: % of subjects with ≥ 3 lines of improvement in distance- corrected near visual
acuity comparing Nyxol + LDP vs placebo alone at 1 hourSecondary:% of subjects with ≥ 2 and ≥ 3 lines gained at time points from 30 min to 6 hours in photopic lighting comparing Nyxol + LDP vs placebo, Nyxol alone, and LDP aloneNo loss of
distance visionPupil diameter at time pointsSafety and tolerability (redness) Visit 1 VEGA-1 Randomization 4 arms 0.75% Nyxol Placebo 17 US sites150presbyopic patients Visit 2(3 – 6 Days Later) Treatment
Arms Nyxol + LDP LDP Drop Nyxol Baseline Nyxol Alone No Treatment Nyxol Baseline LDP Alone LDP Drop Placebo Baseline Placebo Alone No
Treatment Placebo BaselineScreening Evening Dosing (3-4 doses) Males or females ≥ 40 and ≤ 64 years of ageBCDVA of 0.0 LogMAR(20/20 Snellen equivalent) or better in each eye under photopic conditionsDCNVA of 0.4 LogMAR (20/50
Snellen equivalent) or worse in photopic conditions in each eye & binocularly Eligibility Criteria P Phase 2 Enrollment Completed Feb to May 2021 – 150 SubjectsReported Topline Results End of 2Q21
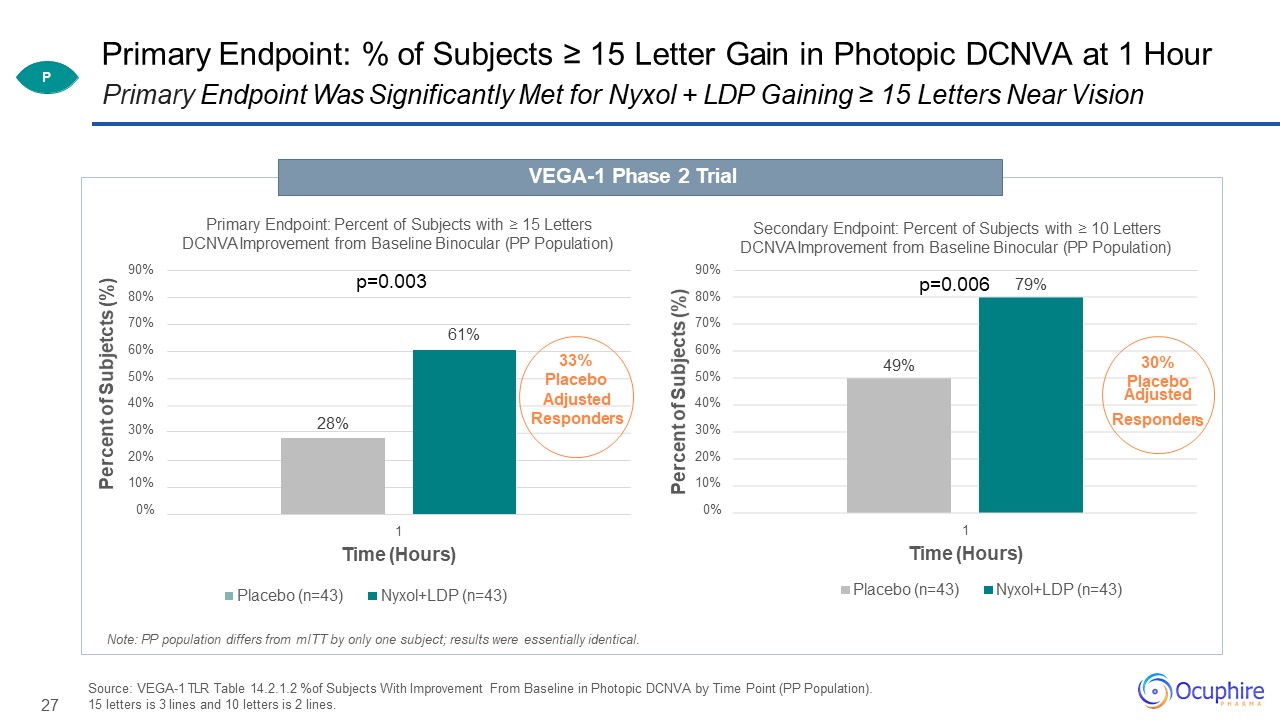
27 79% 90%80%70%60%50%40%30%20%10%0% Percent of Subjects (%) Secondary Endpoint: Percent of
Subjects with ≥ 10 Letters DCNVA Improvement from Baseline Binocular (PP Population) 1Time (Hours)Placebo (n=43) Nyxol+LDP (n=43) Primary Endpoint: % of Subjects ≥ 15 Letter Gain in Photopic DCNVA at 1 HourPrimary Endpoint Was
Significantly Met for Nyxol + LDP Gaining ≥ 15 Letters Near Vision VEGA-1 Phase 2 Trial 61% 28% 90%80%70%60%50%40%30%20%10%0% Percent of Subjetcts (%) Primary Endpoint: Percent of Subjects with ≥ 15 Letters DCNVA Improvement
from Baseline Binocular (PP Population) 1Time (Hours)Placebo (n=43) Nyxol+LDP
(n=43) p=0.003 33%Placebo Adjusted Responders p=0.006 49% 30% PlaceboAdjusted Responder s Source: VEGA-1 TLR Table 14.2.1.2 %of Subjects With Improvement
From Baseline in Photopic DCNVA by Time Point (PP Population). 15 letters is 3 lines and 10 letters is 2 lines. Note: PP population differs from mITT by only one subject; results were essentially identical. P
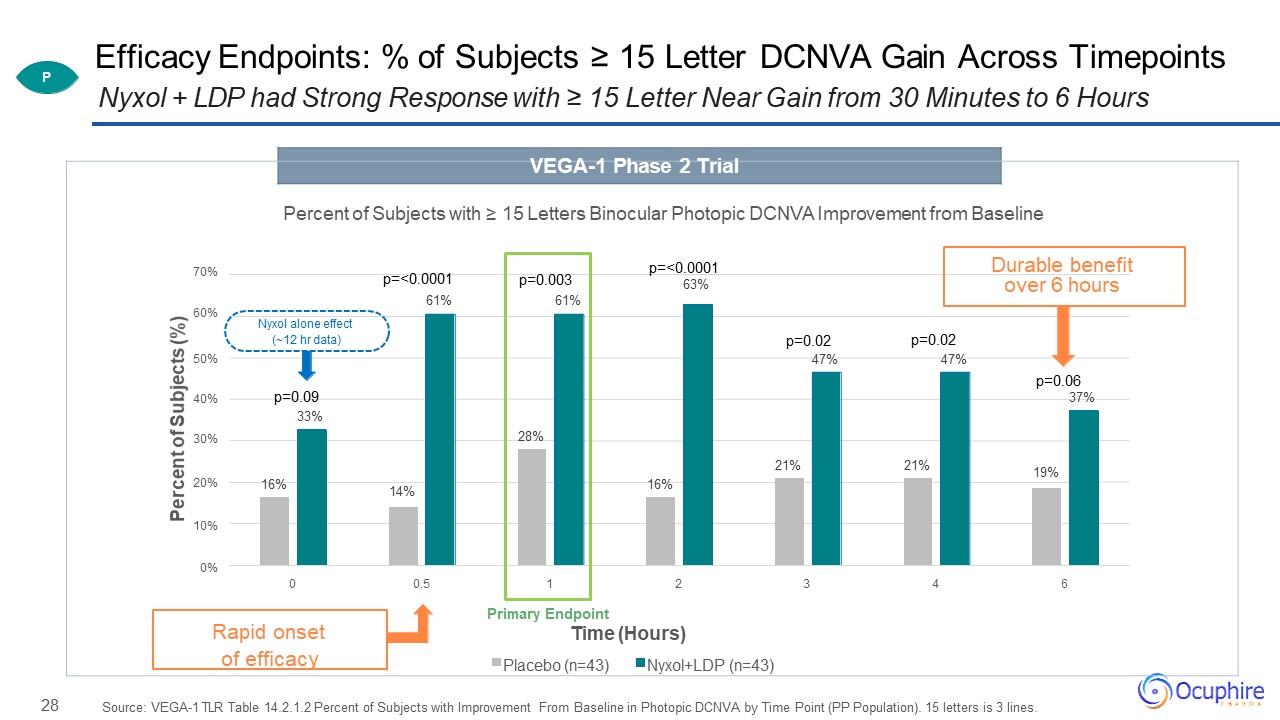
28 Efficacy Endpoints: % of Subjects ≥ 15 Letter DCNVA Gain Across TimepointsNyxol + LDP had Strong
Response with ≥ 15 Letter Near Gain from 30 Minutes to 6 Hours Source: VEGA-1 TLR Table 14.2.1.2 Percent of Subjects with Improvement From Baseline in Photopic DCNVA by Time Point (PP Population). 15 letters is 3
lines. Percent of Subjects (%) VEGA-1 Phase 2 Trial Percent of Subjects with ≥ 15 Letters Binocular Photopic DCNVA Improvement from Baseline70%
p=<0.0001 Durable benefitp=<0.0001 p=0.003 63% over 6 hours61% 61%60%Nyxol alone effect(~12 hr data) p=0.02 p=0.0250% 47% 47%p=0.0640% p=0.09 37%33%30% 28%21% 21% 19%20% 16% 14% 16%10%0%0 0.5 1 2 3 4 6Primary EndpointRapid onset Time
(Hours)of efficacy Placebo (n=43) Nyxol+LDP (n=43) P
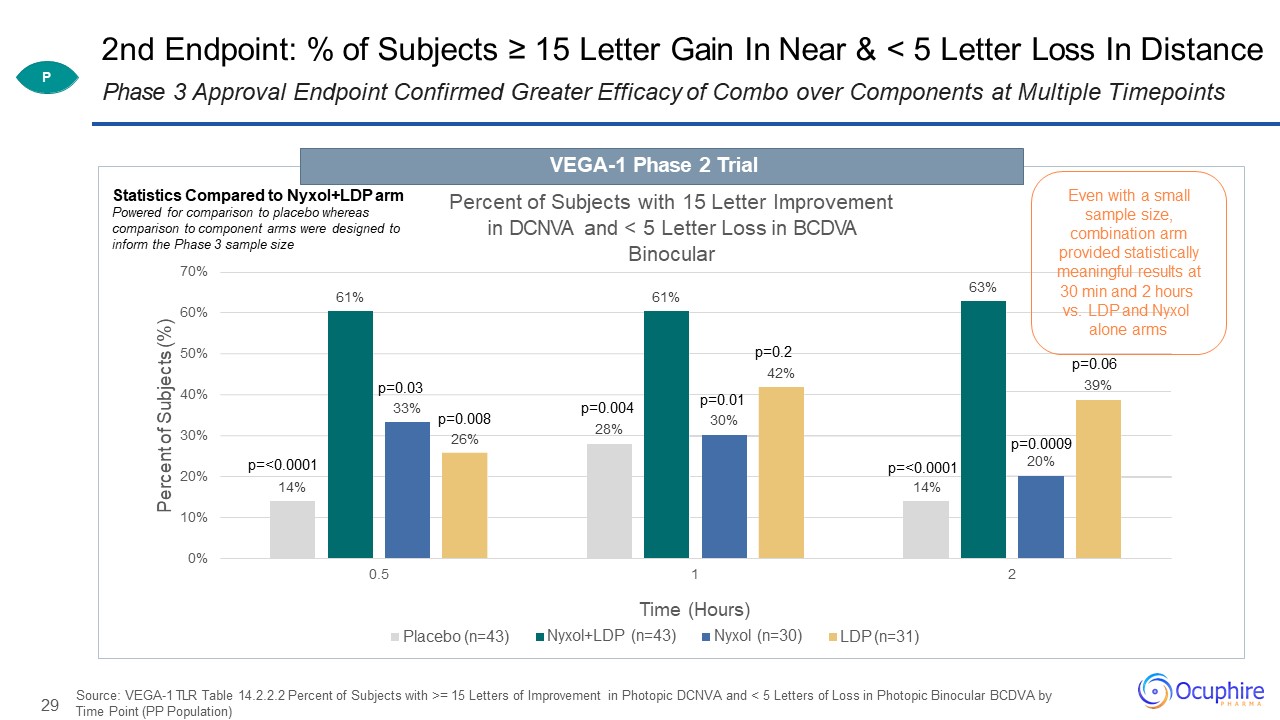
29 2nd Endpoint: % of Subjects ≥ 15 Letter Gain In Near & < 5 Letter Loss In Distance Source:
VEGA-1 TLR Table 14.2.2.2 Percent of Subjects with >= 15 Letters of Improvement in Photopic DCNVA and < 5 Letters of Loss in Photopic Binocular BCDVA by Time Point (PP Population) Phase 3 Approval Endpoint Confirmed Greater Efficacy of
Combo over Components at Multiple Timepoints 14% 14% 61% 61% 63% 33% 26% 42% 10%0% 20% 30% 40% 50% 60% 70% 0.5 1 2 Percent of Subjects (%) Percent of Subjects with 15
Letter Improvement in DCNVA and < 5 Letter Loss in BCDVA Binocular Placebo (n=43) Time (Hours)Nyxol+LDP (n=43) Nyxol (n=30) LDP (n=31) p=<0.0001 p=0.03 p=0.0130% p=0.008 p=0.00428% p=0.06 39%
p=<0.0001 p=0.2 p=0.000920% Statistics Compared to Nyxol+LDP arm Powered for comparison to placebo whereas comparison to component arms were designed to inform the Phase 3 sample size VEGA-1 Phase 2 Trial P Even with a
small sample size, combination arm provided statistically meaningful results at 30 min and 2 hours vs. LDP and Nyxol alone arms
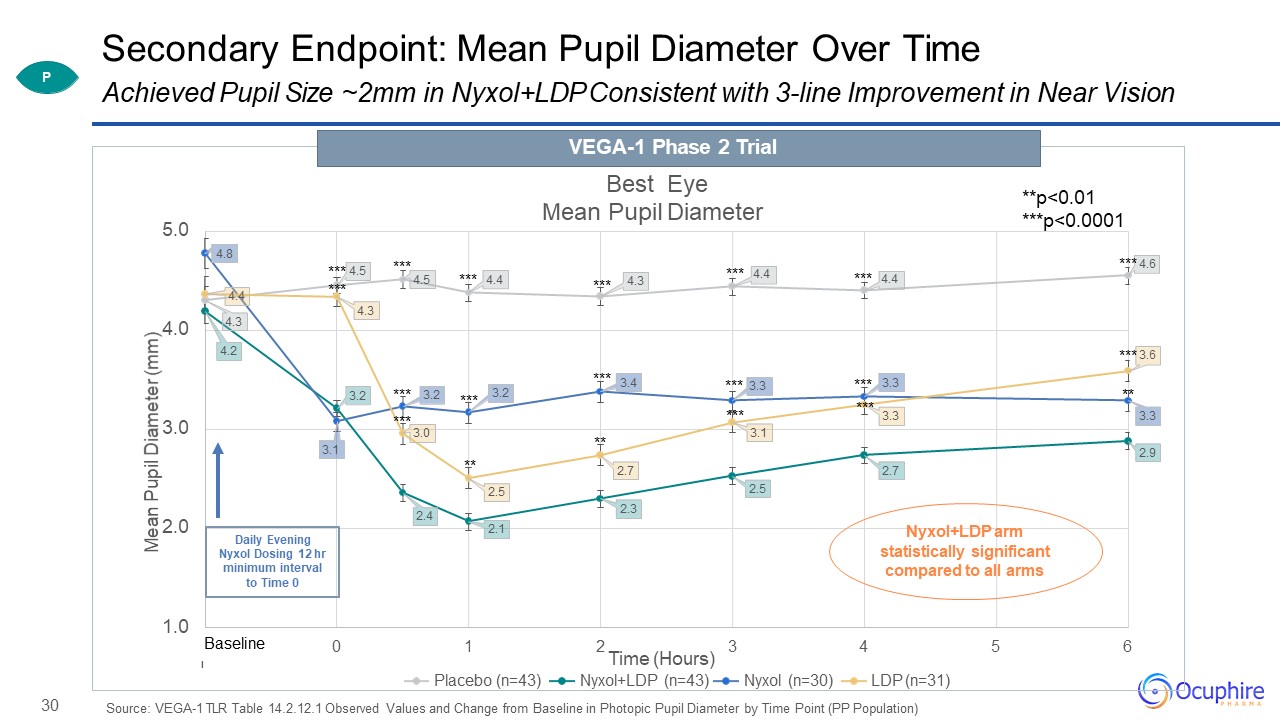
30 Secondary Endpoint:
Mean Pupil Diameter Over Time Source: VEGA-1 TLR Table 14.2.12.1 Observed Values and Change from Baseline in Photopic Pupil Diameter by Time Point (PP
Population) 4.3 4.5 4.4 4.3 4.2 3.2 2.4 2.1 2.3 2.5 2.7 2.9 4.8 3.1 3.2 3.2 3.4 3.3 3.3 4.4 4.3 3.0 2.5 2.7 3.1 3.3 1.0 2.0 3.0 4.0 5.0 -1 0 1 2 3 4 5 6 Mean
Pupil Diameter (mm) Best Eye Mean Pupil Diameter Time (Hours)Placebo (n=43) Nyxol+LDP (n=43) Nyxol (n=30) LDP (n=31) *** 4.5 *** *** ****** *** *** ** *** *** ** *** 4.4 *** 4.4 *** 3.3*** *** 4.6 ** *** 3.6 Daily
Evening Nyxol Dosing 12 hr minimum interval to Time 0 Baseline ****** Nyxol+LDP arm statistically significant compared to all arms **p<0.01***p<0.0001 Achieved Pupil Size ~2mm in Nyxol+LDP Consistent with 3-line
Improvement in Near VisionVEGA-1 Phase 2 Trial P
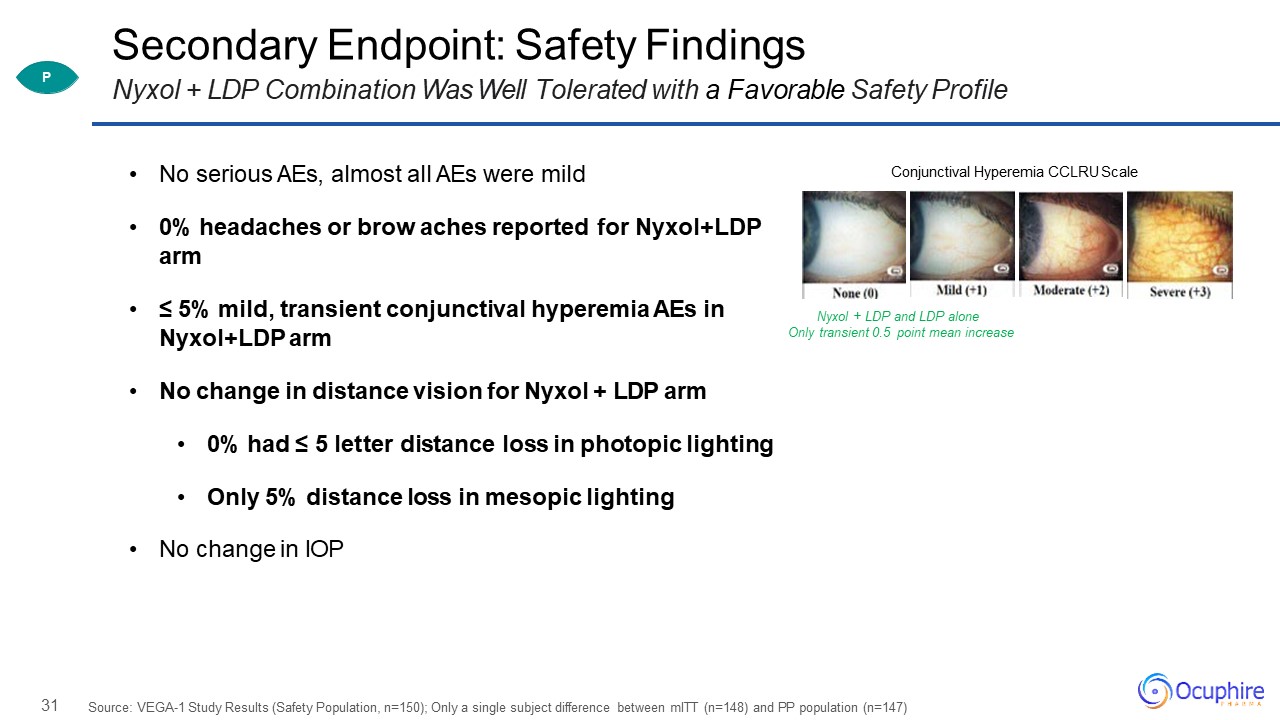
31 Secondary Endpoint: Safety FindingsNyxol + LDP Combination Was Well Tolerated with a Favorable Safety
Profile Source: VEGA-1 Study Results (Safety Population, n=150); Only a single subject difference between mITT (n=148) and PP population (n=147) No serious AEs, almost all AEs were mild0% headaches or brow aches reported for Nyxol+LDP arm ≤
5% mild, transient conjunctival hyperemia AEs in Nyxol+LDP armNo change in distance vision for Nyxol + LDP arm0% had ≤ 5 letter distance loss in photopic lightingOnly 5% distance loss in mesopic lightingNo change in IOP Conjunctival Hyperemia
CCLRU Scale Nyxol + LDP and LDP aloneOnly transient 0.5 point mean increase P
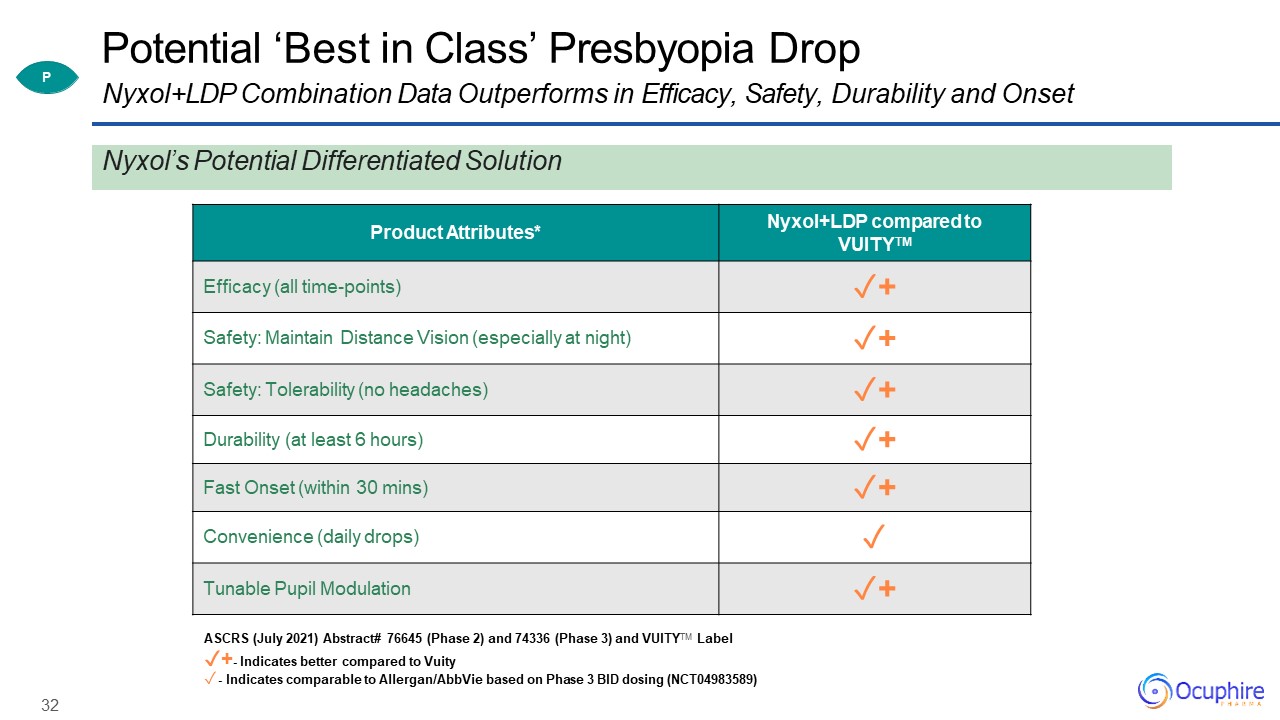
32 Product Attributes* Nyxol+LDP compared to VUITYTM Efficacy (all time-points) ✓+ Safety: Maintain
Distance Vision (especially at night) ✓+ Safety: Tolerability (no headaches) ✓+ Durability (at least 6 hours) ✓+ Fast Onset (within 30 mins) ✓+ Convenience (daily drops) ✓ Tunable Pupil Modulation ✓+ ASCRS (July 2021) Abstract#
76645 (Phase 2) and 74336 (Phase 3) and VUITYTM Label✓+- Indicates better compared to Vuity- Indicates comparable to Allergan/AbbVie based on Phase 3 BID dosing (NCT04983589) Potential ‘Best in Class’ Presbyopia DropNyxol+LDP Combination Data
Outperforms in Efficacy, Safety, Durability and Onset P Nyxol’s Potential Differentiated Solution
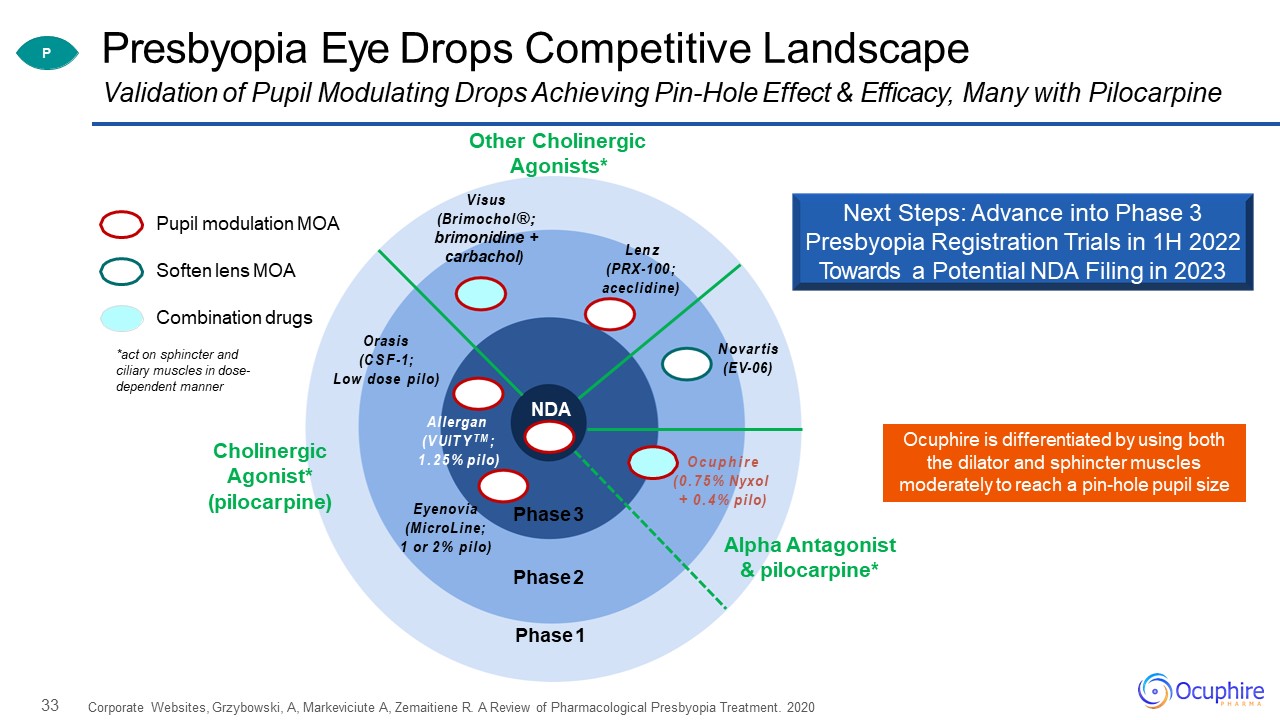
33 Presbyopia Eye Drops Competitive Landscape Corporate Websites, Grzybowski, A, Markeviciute A,
Zemaitiene R. A Review of Pharmacological Presbyopia Treatment. 2020 Validation of Pupil Modulating Drops Achieving Pin-Hole Effect & Efficacy, Many with Pilocarpine Pupil modulation MOA Combination drugs Soften lens MOA Phase
3 Phase 2 Phase 1 Allergan (VUITYTM;1.25% pilo) Orasis (CSF-1;Low dose pilo) Ocuphire (0.75% Nyxol+ 0.4% pilo) carbachol) Other Cholinergic Agonists*Visus (Brimochol®; brimonidine + Cholinergic Agonist*
(pilocarpine) Lenz (PRX-100;aceclidine) Eyenovia (MicroLine; 1 or 2% pilo) Novartis (EV-06) Alpha Antagonist & pilocarpine* P NDA *act on sphincter and ciliary muscles in dose- dependent manner Ocuphire is
differentiated by using both the dilator and sphincter muscles moderately to reach a pin-hole pupil size Next Steps: Advance into Phase 3 Presbyopia Registration Trials in 1H 2022 Towards a Potential NDA Filing in 2023
34 Nyxol® Phentolamine Mesylate NVD P Presbyopia RM Night Vision
Disturbances Reversal of Mydriasis 0.4% +
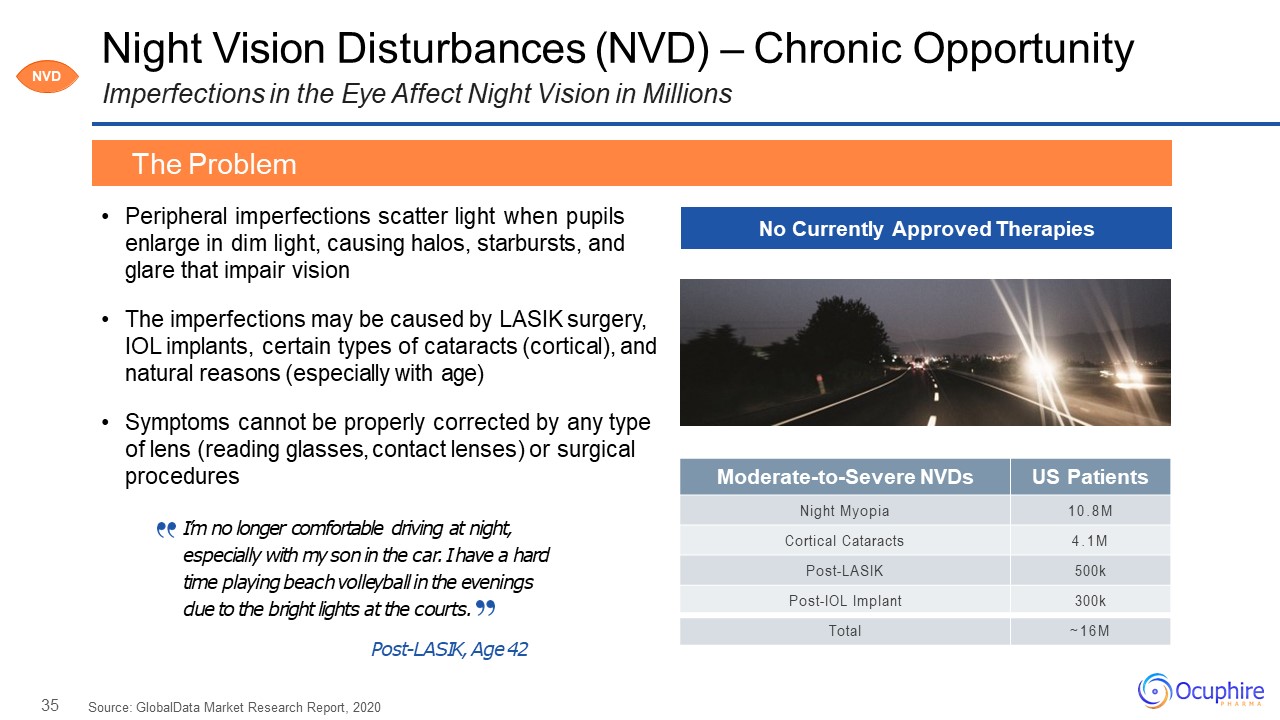
35 Moderate-to-Severe NVDs US Patients Night Myopia 10.8M Cortical
Cataracts 4.1M Post-LASIK 500k Post-IOL Implant 300k Total ~16M Night Vision Disturbances (NVD) – Chronic OpportunityImperfections in the Eye Affect Night Vision in Millions Peripheral imperfections scatter light when pupils enlarge in
dim light, causing halos, starbursts, and glare that impair visionThe imperfections may be caused by LASIK surgery, IOL implants, certain types of cataracts (cortical), and natural reasons (especially with age)Symptoms cannot be properly
corrected by any type of lens (reading glasses, contact lenses) or surgical procedures Source: GlobalData Market Research Report, 2020 The Problem No Currently Approved Therapies NVD I’m no longer comfortable driving at night,
especially with my son in the car. I have a hard time playing beach volleyball in the evenings due to the bright lights at the courts.Post-LASIK, Age 42
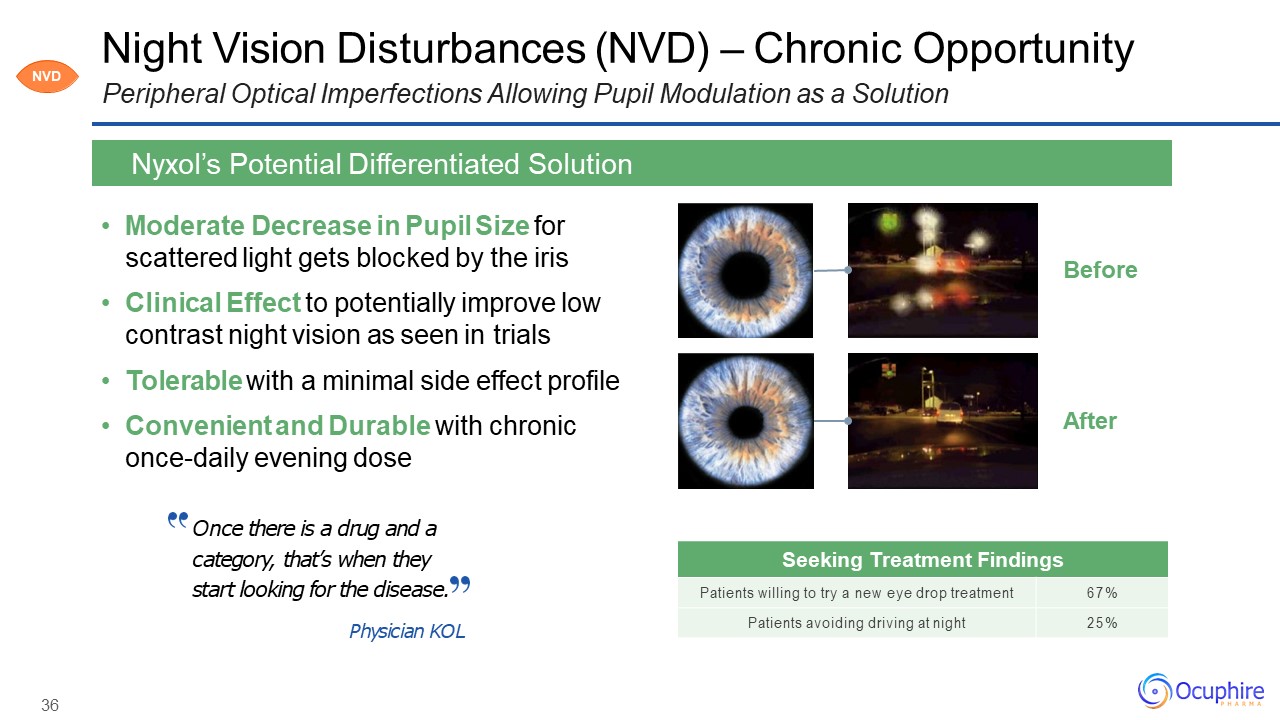
36 Night Vision Disturbances (NVD) – Chronic OpportunityPeripheral Optical Imperfections Allowing Pupil
Modulation as a Solution Moderate Decrease in Pupil Size for scattered light gets blocked by the irisClinical Effect to potentially improve low contrast night vision as seen in trialsTolerable with a minimal side effect profileConvenient and
Durable with chronic once-daily evening dose Nyxol’s Potential Differentiated Solution After Before Once there is a drug and a category, that’s when they start looking for the disease.Physician KOL Seeking Treatment
Findings Patients willing to try a new eye drop treatment 67% Patients avoiding driving at night 25% NVD
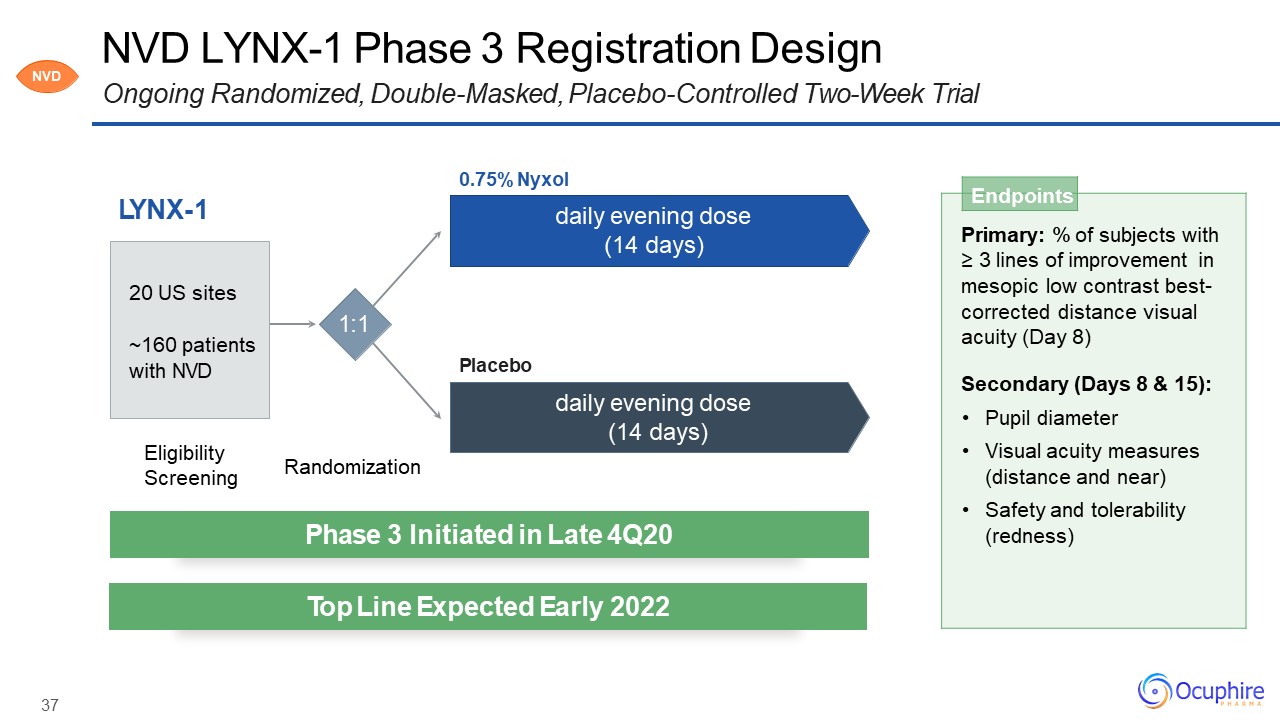
37 NVD LYNX-1 Phase 3 Registration DesignOngoing Randomized, Double-Masked, Placebo-Controlled
Two-Week Trial LYNX-1 Endpoints Primary: % of subjects with≥ 3 lines of improvement in mesopic low contrast best- corrected distance visual acuity (Day 8)Secondary (Days 8 & 15):Pupil diameterVisual acuity measures (distance
and near)Safety and tolerability (redness) Eligibility Screening Randomization 1:1 0.75% Nyxoldaily evening dose (14 days) daily evening dose (14 days) Placebo 20 US sites~160 patients with NVD Phase 3 Initiated in
Late 4Q20 Top Line Expected Early 2022 NVD
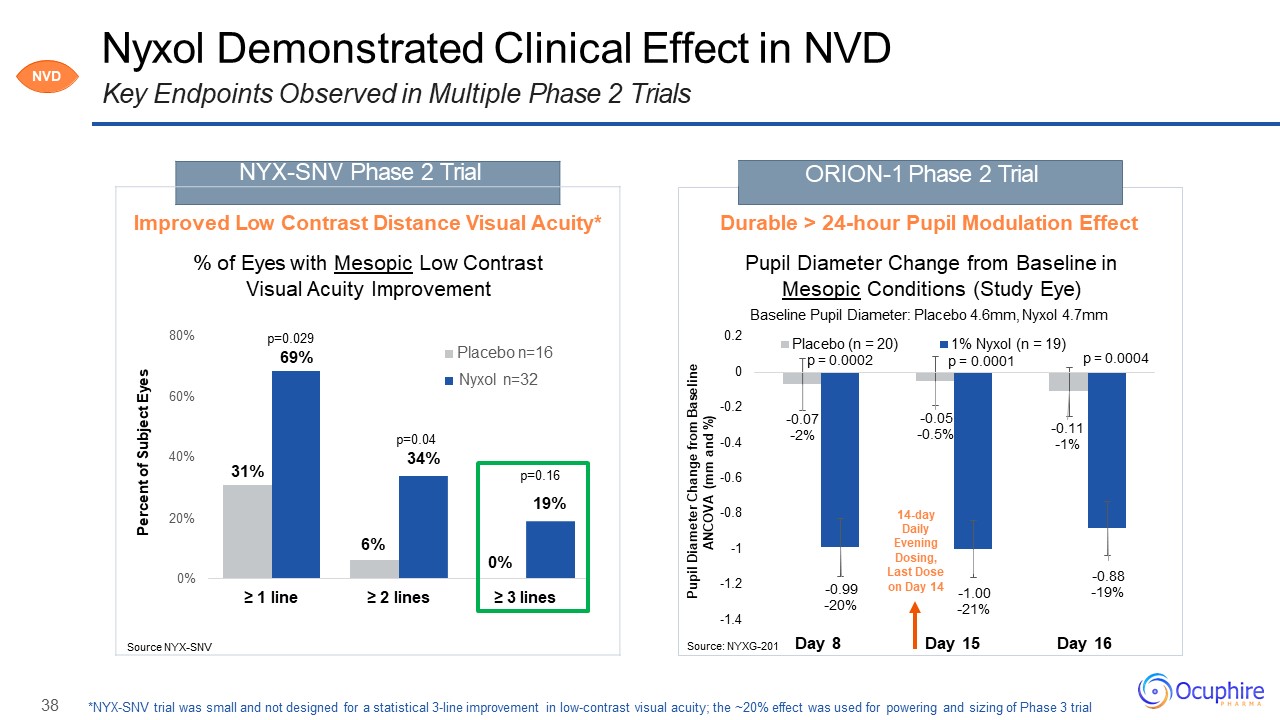
38 -0.07-2% -0.05-0.5% -0.11-1% -0.99-20% -1.00-21% -0.88-19% -1.4 -1.2 -1 -0.8 -0.6 -0.4 -0.2 0 0.2 Day
8 Day 15 Day 16 Pupil Diameter Change from Baseline ANCOVA (mm and %) Placebo (n = 20) 14-day Daily Evening Dosing, Last Dose on Day 14 ORION-1 Phase 2 Trial Nyxol Demonstrated Clinical Effect in NVDKey Endpoints Observed
in Multiple Phase 2 Trials Source: NYXG-201 Durable > 24-hour Pupil Modulation EffectPupil Diameter Change from Baseline in Mesopic Conditions (Study Eye)Baseline Pupil Diameter: Placebo 4.6mm, Nyxol 4.7mm Percent of Subject
Eyes NYX-SNV Phase 2 Trial Improved Low Contrast Distance Visual Acuity*% of Eyes with Mesopic Low Contrast Visual Acuity Improvement80% p=0.02969% Placebo n=16Nyxol n=3260%p=0.0440% 34%31% p=0.1619%20%6%0%0%≥ 1 line ≥ 2 lines ≥ 3
linesSource NYX-SNV *NYX-SNV trial was small and not designed for a statistical 3-line improvement in low-contrast visual acuity; the ~20% effect was used for powering and sizing of Phase 3 trial p = 0.0002 1% Nyxol (n = 19)p =
0.0001 p = 0.0004 NVD
39 APX3330 APX3330 DR Diabetic Retinopathy DME Diabetic Macular Edema wAMD Wet
Age-Related Macular Degeneration
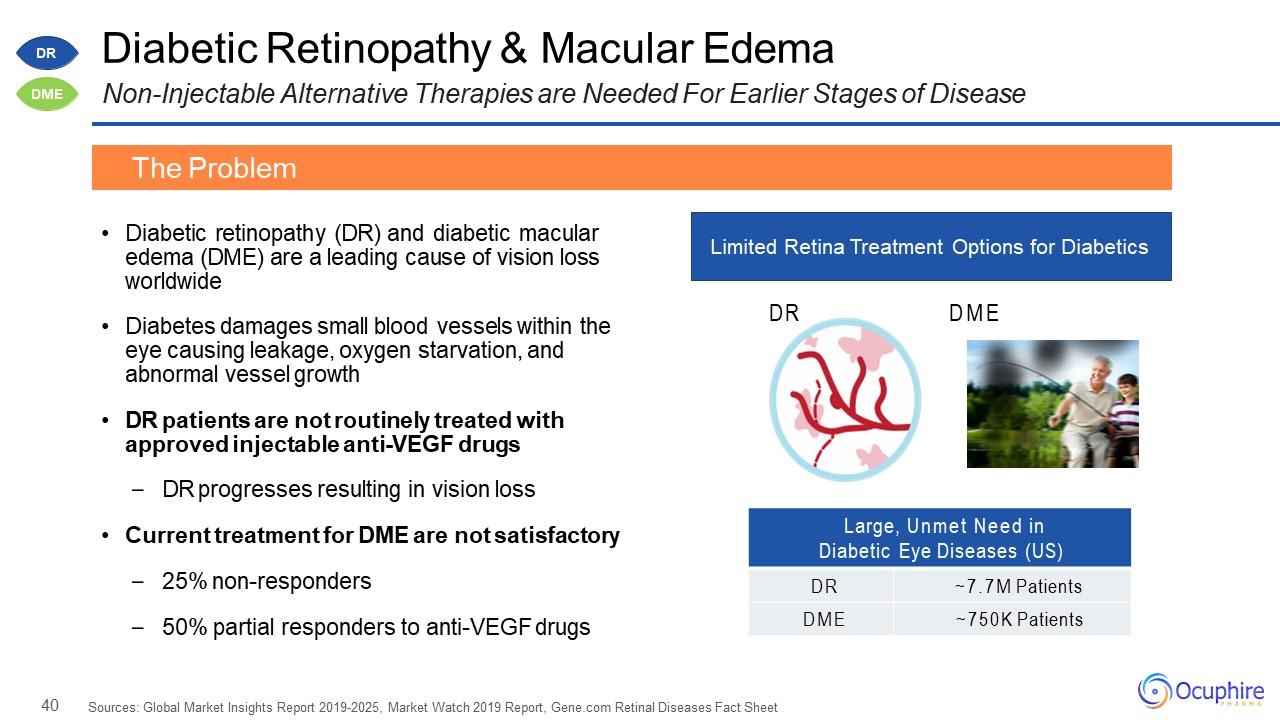
40 Large, Unmet Need in Diabetic Eye Diseases (US) DR ~7.7M Patients DME ~750K Patients Diabetic
Retinopathy & Macular EdemaNon-Injectable Alternative Therapies are Needed For Earlier Stages of Disease Diabetic retinopathy (DR) and diabetic macular edema (DME) are a leading cause of vision loss worldwideDiabetes damages small blood
vessels within the eye causing leakage, oxygen starvation, and abnormal vessel growthDR patients are not routinely treated with approved injectable anti-VEGF drugsDR progresses resulting in vision lossCurrent treatment for DME are not
satisfactory25% non-responders50% partial responders to anti-VEGF drugs Sources: Global Market Insights Report 2019-2025, Market Watch 2019 Report, Gene.com Retinal Diseases Fact Sheet The Problem Limited Retina Treatment Options for
Diabetics DR DME DR DME
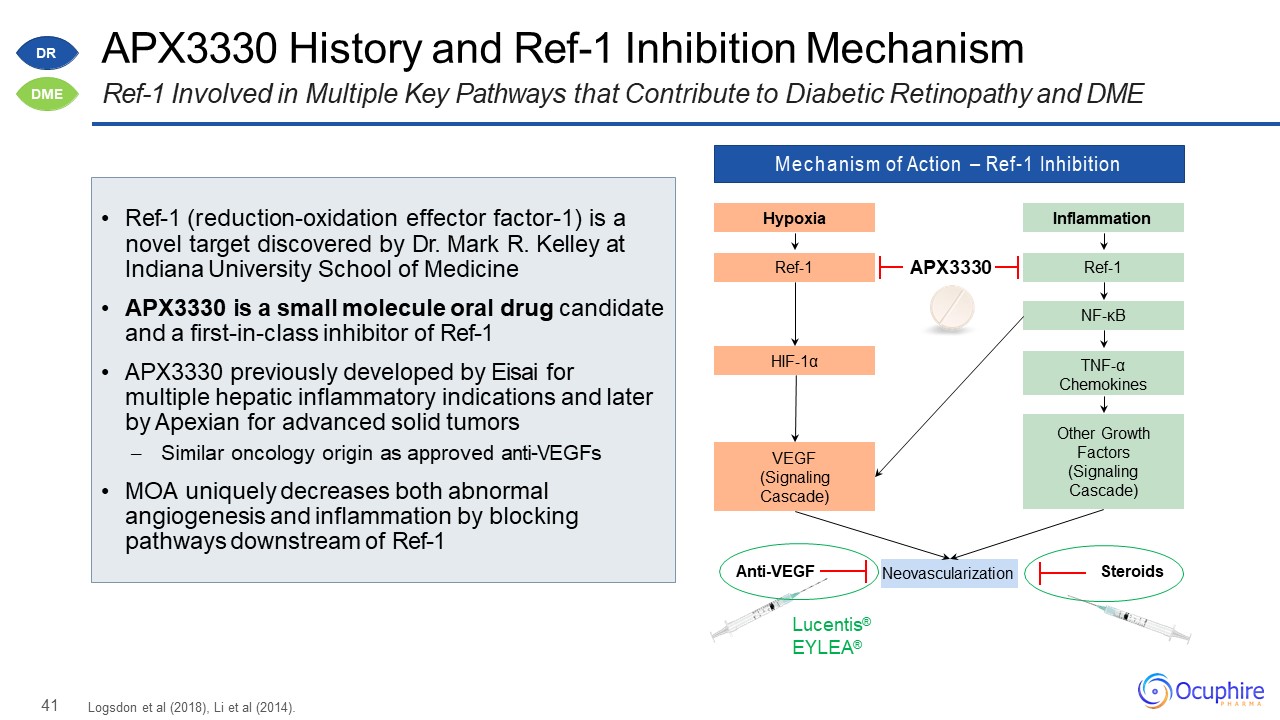
41 APX3330 History and Ref-1 Inhibition MechanismRef-1 Involved in Multiple Key Pathways that Contribute
to Diabetic Retinopathy and DME Mechanism of Action – Ref-1 Inhibition Hypoxia Ref-1 HIF-1α VEGF(Signaling Cascade) Inflammation Ref-1 NF-κB Other Growth Factors (Signaling
Cascade) TNF-αChemokines Neovascularization Lucentis® EYLEA® Anti-VEGF Steroids APX3330 Logsdon et al (2018), Li et al (2014). Ref-1 (reduction-oxidation effector factor-1) is a
novel target discovered by Dr. Mark R. Kelley at Indiana University School of MedicineAPX3330 is a small molecule oral drug candidate and a first-in-class inhibitor of Ref-1APX3330 previously developed by Eisai for multiple hepatic inflammatory
indications and later by Apexian for advanced solid tumors– Similar oncology origin as approved anti-VEGFsMOA uniquely decreases both abnormal angiogenesis and inflammation by blocking pathways downstream of Ref-1 DR DME
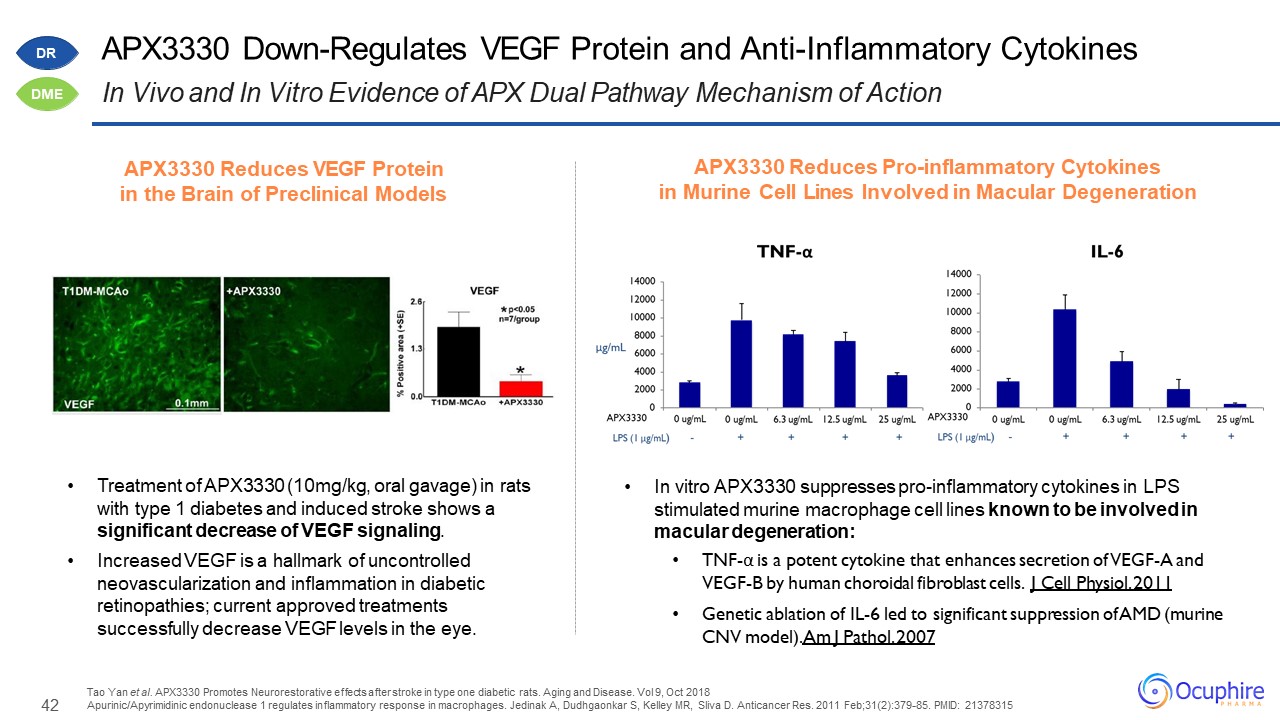
42 APX3330 Down-Regulates VEGF Protein and Anti-Inflammatory Cytokines In Vivo and In Vitro Evidence of
APX Dual Pathway Mechanism of Action Treatment of APX3330 (10mg/kg, oral gavage) in rats with type 1 diabetes and induced stroke shows a significant decrease of VEGF signaling.Increased VEGF is a hallmark of uncontrolled neovascularization and
inflammation in diabetic retinopathies; current approved treatments successfully decrease VEGF levels in the eye. Tao Yan et al. APX3330 Promotes Neurorestorative effects after stroke in type one diabetic rats. Aging and Disease. Vol 9, Oct
2018Apurinic/Apyrimidinic endonuclease 1 regulates inflammatory response in macrophages. Jedinak A, Dudhgaonkar S, Kelley MR, Sliva D. Anticancer Res. 2011 Feb;31(2):379-85. PMID: 21378315 In vitro APX3330 suppresses pro-inflammatory cytokines
in LPS stimulated murine macrophage cell lines known to be involved in macular degeneration: TNF-α is a potent cytokine that enhances secretion of VEGF-A and VEGF-B by human choroidal fibroblast cells. J Cell Physiol. 2011Genetic ablation of
IL-6 led to significant suppression of AMD (murine CNV model). Am J Pathol. 2007 APX3330 Reduces VEGF Protein in the Brain of Preclinical Models APX3330 Reduces Pro-inflammatory Cytokinesin Murine Cell Lines Involved in Macular
Degeneration DR DME
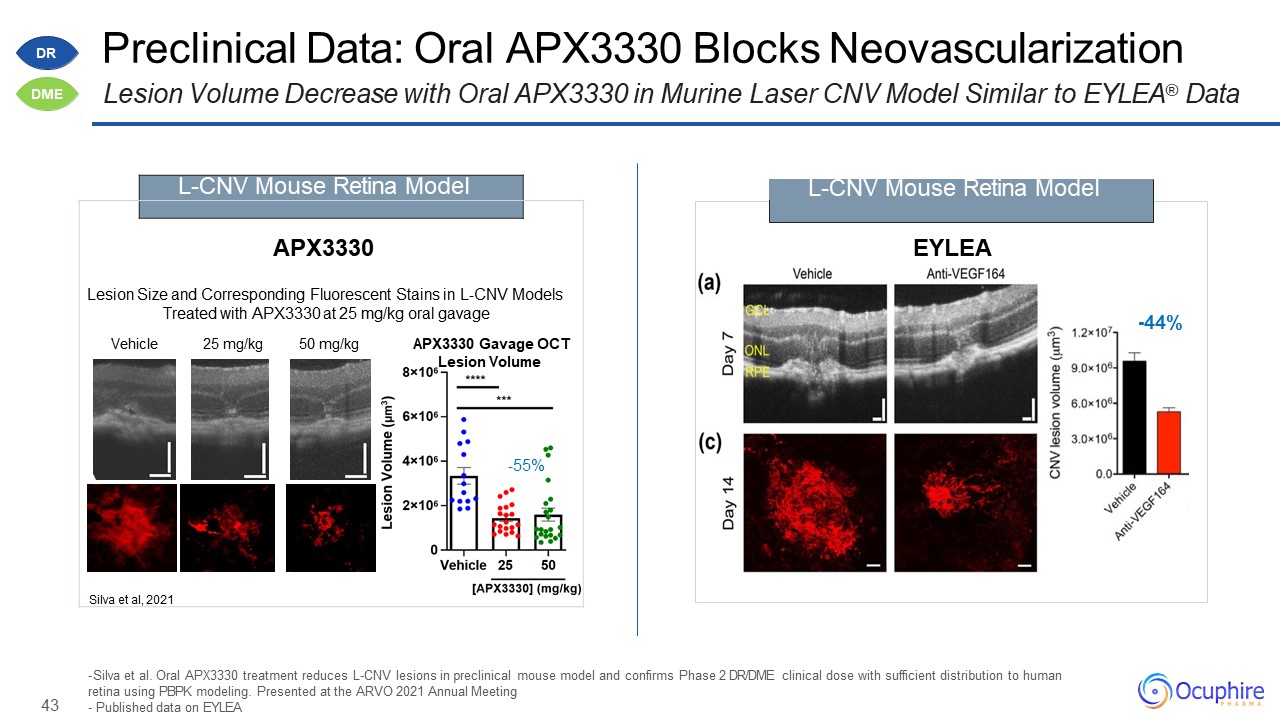
43 Preclinical Data: Oral APX3330 Blocks NeovascularizationLesion Volume Decrease with Oral APX3330 in
Murine Laser CNV Model Similar to EYLEA® Data Silva et al. Oral APX3330 treatment reduces L-CNV lesions in preclinical mouse model and confirms Phase 2 DR/DME clinical dose with sufficient distribution to human retina using PBPK modeling.
Presented at the ARVO 2021 Annual MeetingPublished data on EYLEA -44% EYLEA L-CNV Mouse Retina Model APX3330Lesion Size and Corresponding Fluorescent Stains in L-CNV Models Treated with APX3330 at 25 mg/kg oral gavageVehicle
25 mg/kg 50 mg/kg APX3330 Gavage OCTLesion Volume-55%Silva et al, 2021 L-CNV Mouse Retina Model DR DME
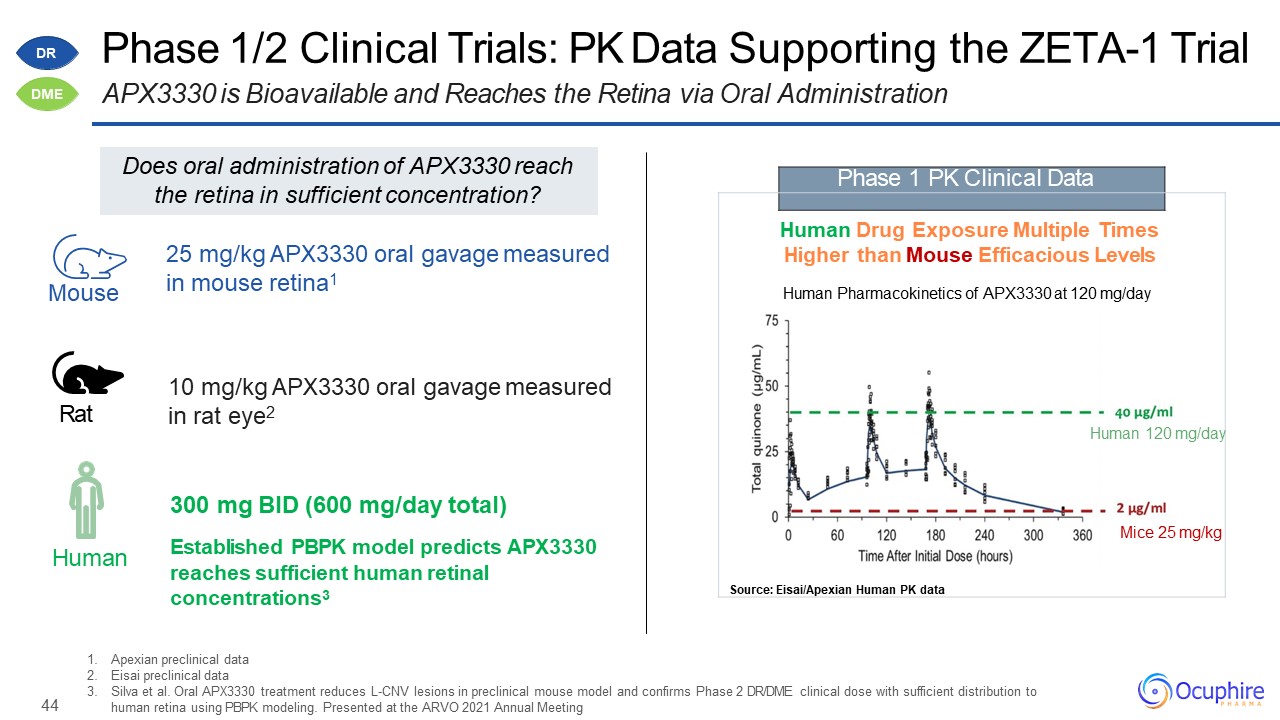
44 Apexian preclinical dataEisai preclinical dataSilva et al. Oral APX3330 treatment reduces L-CNV
lesions in preclinical mouse model and confirms Phase 2 DR/DME clinical dose with sufficient distribution to human retina using PBPK modeling. Presented at the ARVO 2021 Annual Meeting Phase 1 PK Clinical Data Human Drug Exposure
Multiple Times Higher than Mouse Efficacious LevelsHuman Pharmacokinetics of APX3330 at 120 mg/dayHuman 120 mg/dayMice 25 mg/kgSource: Eisai/Apexian Human PK data Human Mouse Rat 10 mg/kg APX3330 oral gavage measured in rat
eye2 300 mg BID (600 mg/day total)Established PBPK model predicts APX3330 reaches sufficient human retinal concentrations3 25 mg/kg APX3330 oral gavage measured in mouse retina1 Phase 1/2 Clinical Trials: PK Data Supporting the ZETA-1
TrialAPX3330 is Bioavailable and Reaches the Retina via Oral Administration Does oral administration of APX3330 reach the retina in sufficient concentration? DR DME
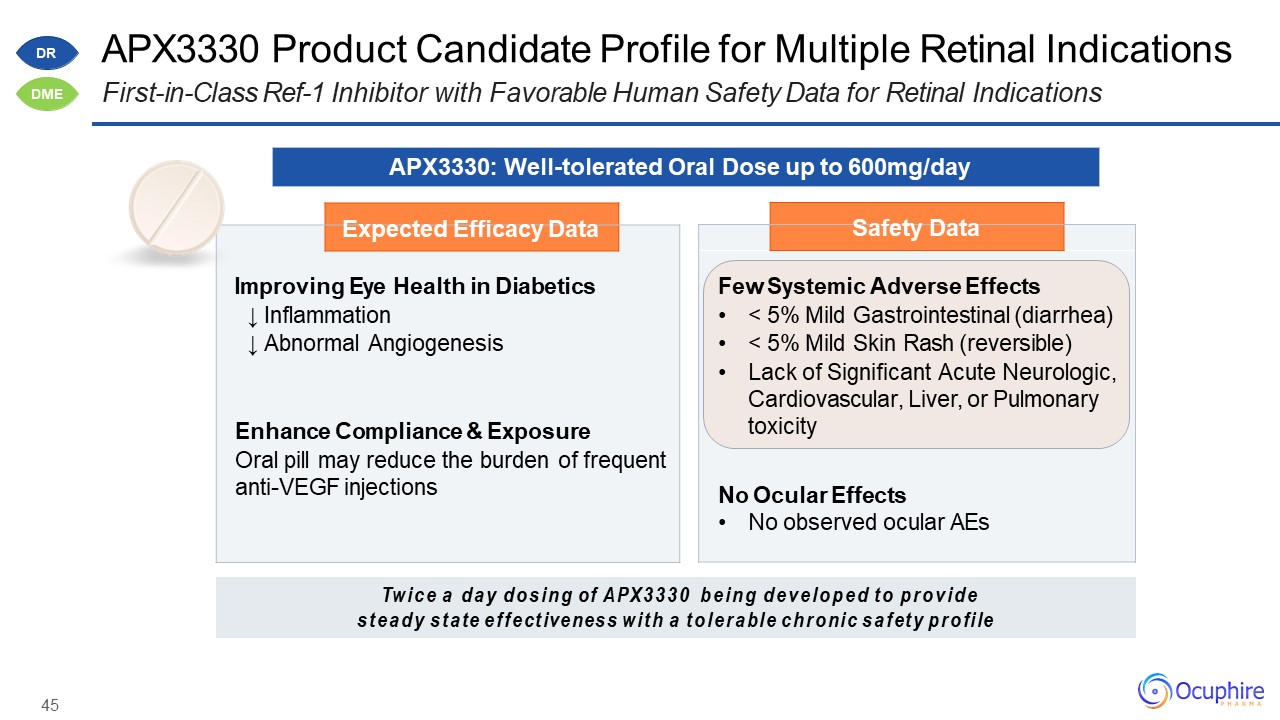
45 APX3330 Product Candidate Profile for Multiple Retinal IndicationsFirst-in-Class Ref-1
Inhibitor with Favorable Human Safety Data for Retinal Indications Expected Efficacy Data Improving Eye Health in Diabetics↓ Inflammation↓ Abnormal AngiogenesisEnhance Compliance & ExposureOral pill may reduce the burden of
frequent anti-VEGF injections Safety Data Few Systemic Adverse Effects< 5% Mild Gastrointestinal (diarrhea)< 5% Mild Skin Rash (reversible)Lack of Significant Acute Neurologic, Cardiovascular, Liver, or Pulmonary
toxicityNo Ocular EffectsNo observed ocular AEs APX3330: Well-tolerated Oral Dose up to 600mg/day Twice a day dosing of APX3330 being developed to provide steady state effectiveness with a tolerable chronic safety
profile DR DME
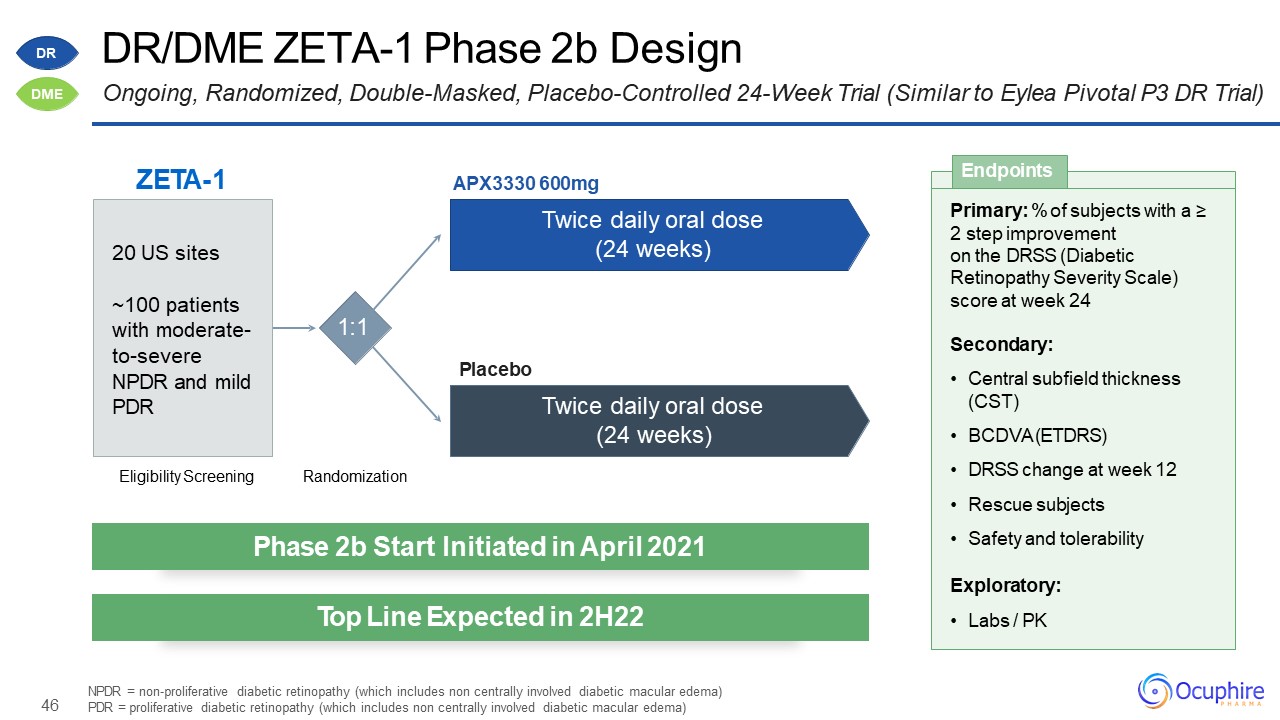
46 DR/DME ZETA-1 Phase 2b DesignOngoing, Randomized, Double-Masked, Placebo-Controlled 24-Week Trial
(Similar to Eylea Pivotal P3 DR Trial) NPDR = non-proliferative diabetic retinopathy (which includes non centrally involved diabetic macular edema) PDR = proliferative diabetic retinopathy (which includes non centrally involved diabetic
macular edema) ZETA-1 Eligibility Screening Randomization 1:1 APX3330 600mgTwice daily oral dose (24 weeks) Twice daily oral dose (24 weeks) Placebo 20 US sites~100 patients with moderate- to-severe NPDR and mild
PDR Primary: % of subjects with a ≥2 step improvement on the DRSS (DiabeticRetinopathy Severity Scale) score at week 24Secondary:Central subfield thickness (CST)BCDVA (ETDRS)DRSS change at week 12Rescue subjectsSafety and
tolerabilityExploratory:Labs / PK Endpoints Phase 2b Start Initiated in April 2021 Top Line Expected in 2H22 DR DME
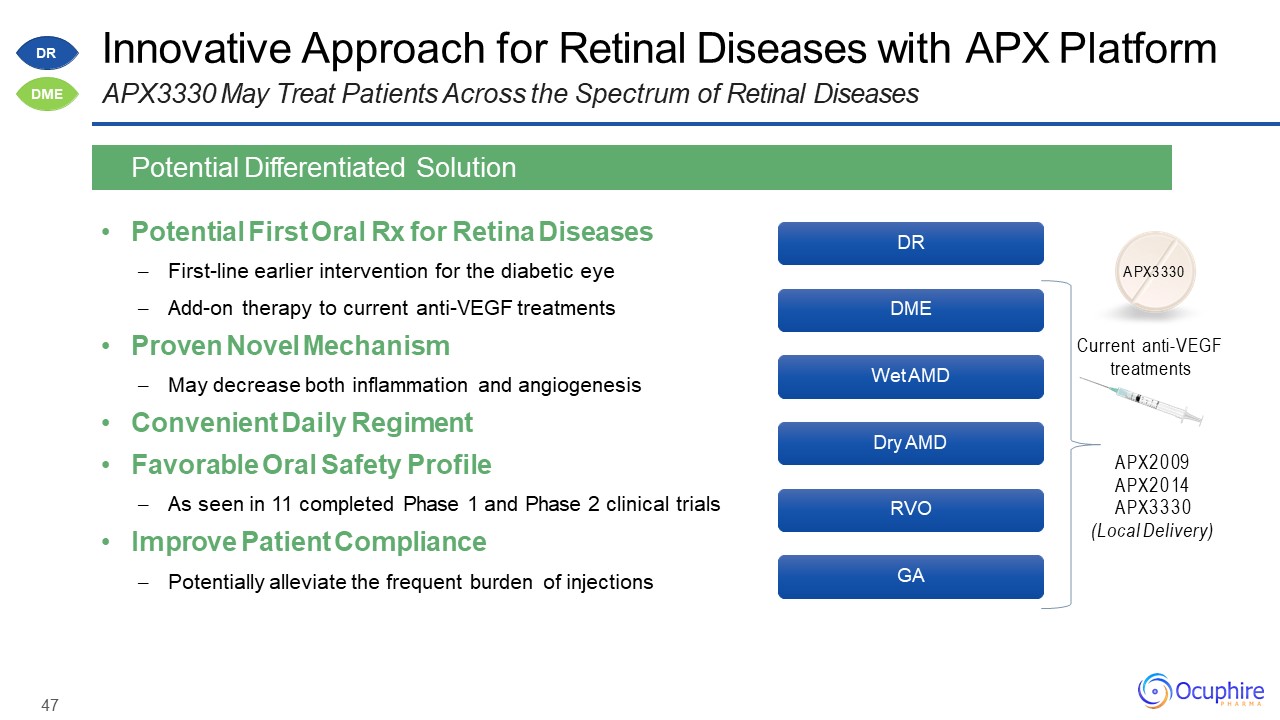
47 Innovative Approach for Retinal Diseases with APX PlatformAPX3330 May Treat Patients Across the
Spectrum of Retinal Diseases Potential First Oral Rx for Retina DiseasesFirst-line earlier intervention for the diabetic eyeAdd-on therapy to current anti-VEGF treatmentsProven Novel MechanismMay decrease both inflammation and
angiogenesisConvenient Daily RegimentFavorable Oral Safety ProfileAs seen in 11 completed Phase 1 and Phase 2 clinical trialsImprove Patient CompliancePotentially alleviate the frequent burden of injections Potential Differentiated
Solution DR DME Wet AMD Dry AMD RVO GA APX3330 Current anti-VEGF treatments APX2009 APX2014 APX3330(Local Delivery) DR DME

48 Boards and Milestones

49 Ed Holland, MDLoyola University Chicago Jay Pepose, MD, PhD UCLA Thomas Samuelson, MD University of
Minnesota Paul Karpecki, OD Indiana University Eliot Lazar, MDGeorgetown University Marguerite McDonald, MD Columbia University David Boyer, MD Chicago Medical School Gerald Horn, MD University of IllinoisCo-Founder Ocularis/Nyxol Past MAB
Member Mark Kelley, PhD Indiana UniversityCo-Founder Apexian/APX3330 Ocuphire's World-Class Medical Advisory BoardFortunate for the Insights of Leading KOLs & Drug Candidate Co-Founders elCON Medical Michael Allingham, MD, PhD
University of North Carolina Peter Kaiser, MD Harvard Medical School Jeffrey Heier, MD Boston University Y. Ralph Chu, MD Northwestern University Douglas Devries, OD University of Nevada David Brown, MD Baylor University Richard
Lindstrom, MD University of Minnesota

50 Ocuphire Board of DirectorsSeasoned Directors with Decades of Drug Development,
M&A/Financings, and Ophthalmology Sean Ainsworth, MBALead Independent Director,Board Director James Manuso, PhD/MBABoard Director Jay Pepose, MD, PhDBoard Director Richard Rodgers, MBABoard Director Susan
Benton, MBABoard Director Cam Gallagher, MBAChair, Board Director Mina Sooch, MBA Vice-Chair, Board DirectorPresident & CEO

51 2021 to 2022 Ocuphire Cadence of MilestonesMultiple Data Catalysts On Path To
NDA(s) Ongoing Partnering Discussions with Leading Ophthalmic Companies (including European and Asian Players) 2021 2022 Report Phase 3 Data for NVD Report 2nd Phase 3 Data for RM Report Pediatric Data in
RM Submit Nyxol NDA for RM Report Phase 2 Data for DR/DME Initiate Two Phase 3 Presbyopia Trials Report Positive Phase 3 Data for RM (MIRA-2) Report Positive Phase 2 Data for Presbyopia (VEGA-1) New Patent
Claims for Presbyopia ASCRS 2021 Presentation for MIRA-2 & VEGA-1 Manufacture 3xRegistration Batches for Nyxol Blow-Fill-Seal (BFS) Eye Drops Initiate 2nd Phase 3 RM and Pediatric RM trial Initiate Phase 3 Chronic Safety Trial Early
2022
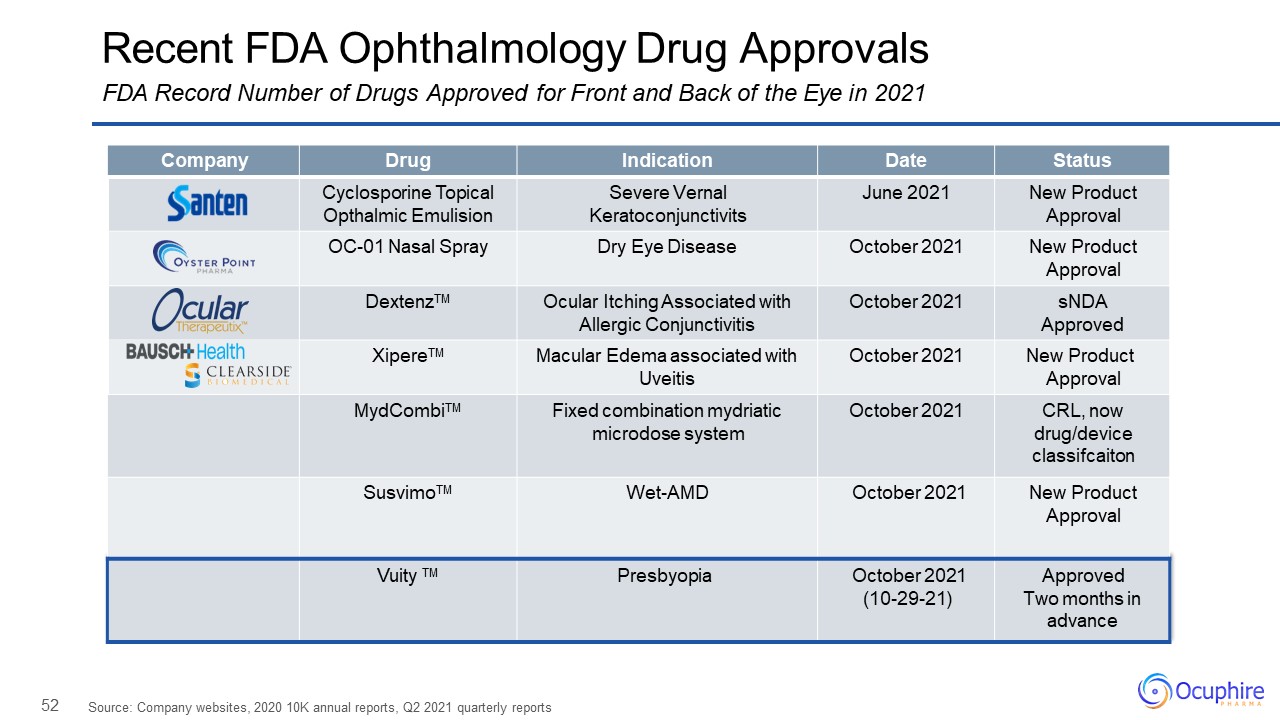
52 Recent FDA Ophthalmology Drug ApprovalsFDA Record Number of Drugs Approved for Front and Back of the
Eye in 2021 Source: Company websites, 2020 10K annual reports, Q2 2021 quarterly reports Company Drug Indication Date Status Cyclosporine Topical Opthalmic Emulision Severe Vernal Keratoconjunctivits June 2021 New Product
Approval OC-01 Nasal Spray Dry Eye Disease October 2021 New Product Approval DextenzTM Ocular Itching Associated with Allergic Conjunctivitis October 2021 sNDAApproved XipereTM Macular Edema associated with Uveitis October
2021 New Product Approval MydCombiTM Fixed combination mydriatic microdose system October 2021 CRL, now drug/device classifcaiton SusvimoTM Wet-AMD October 2021 New Product Approval Vuity TM Presbyopia October
2021(10-29-21) Approved Two months inadvance
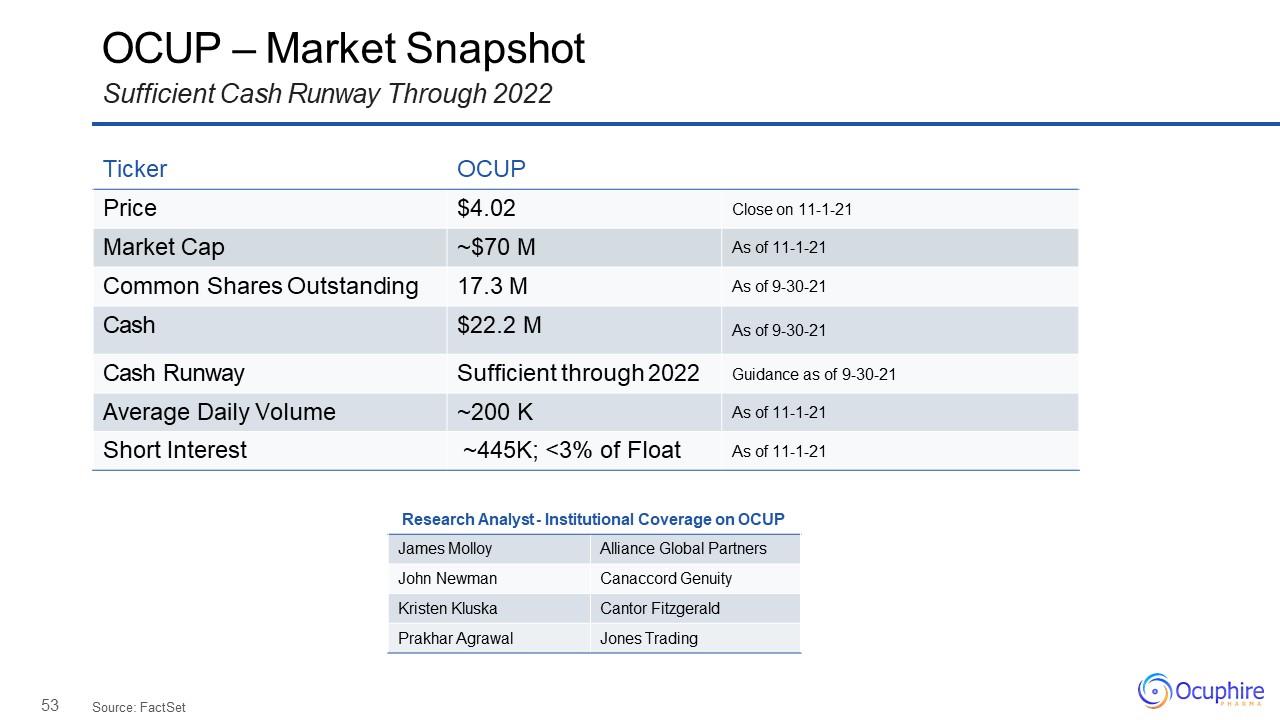
53 OCUP – Market SnapshotSufficient Cash Runway Through 2022 Source:
FactSet Ticker OCUP Price $4.02 Close on 11-1-21 Market Cap ~$70 M As of 11-1-21 Common Shares Outstanding 17.3 M As of 9-30-21 Cash $22.2 M As of 9-30-21 Cash Runway Sufficient through 2022 Guidance as of 9-30-21 Average
Daily Volume ~200 K As of 11-1-21 Short Interest ~445K; <3% of Float As of 11-1-21 Research Analyst - Institutional Coverage on OCUP James Molloy Alliance Global Partners John Newman Canaccord Genuity Kristen Kluska Cantor
Fitzgerald Prakhar Agrawal Jones Trading

Restore Vision & Clarity www.ocuphire.com ir@ocuphire.com
55 Appendix
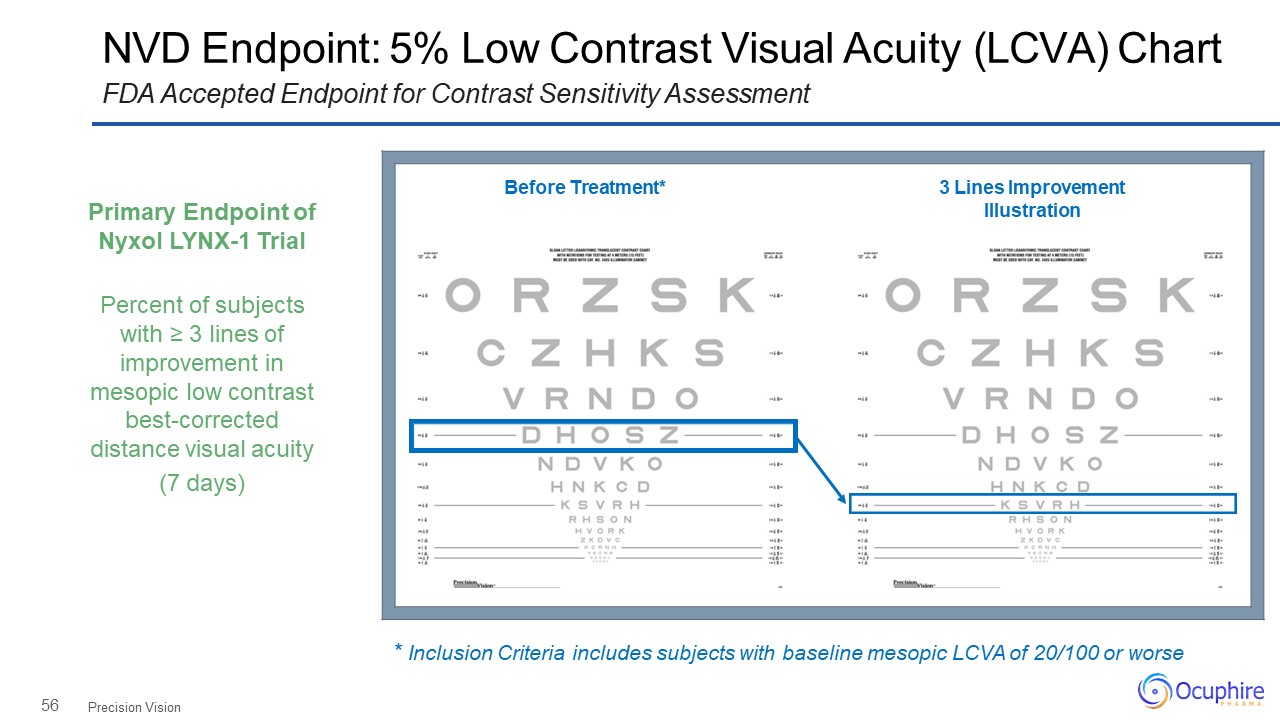
56 NVD Endpoint: 5% Low Contrast Visual Acuity (LCVA) ChartFDA Accepted Endpoint for Contrast
Sensitivity Assessment Precision Vision Before Treatment* 3 Lines Improvement Illustration Primary Endpoint of Nyxol LYNX-1 TrialPercent of subjects with ≥ 3 lines of improvement in mesopic low contrast best-corrected
distance visual acuity(7 days) * Inclusion Criteria includes subjects with baseline mesopic LCVA of 20/100 or worse
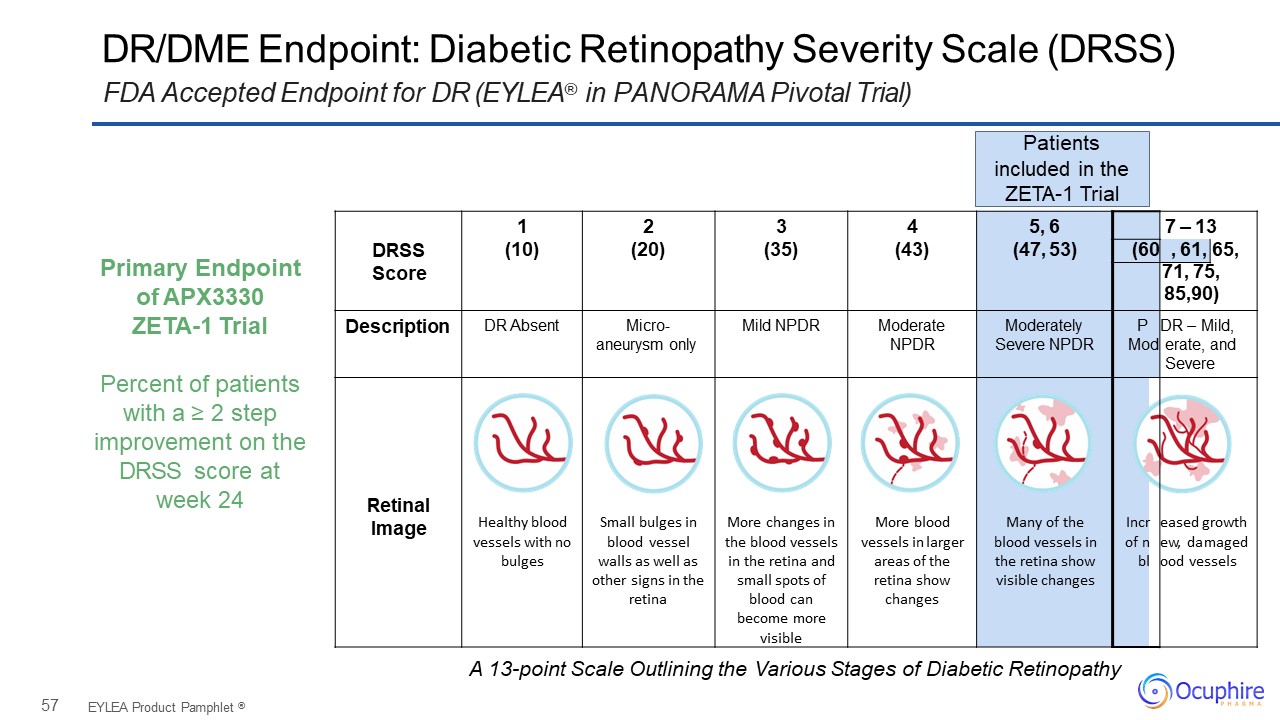
57 DRSSScore 1(10) 2(20) 3(35) 4(43) 5, 6(47, 53) 7 – 13 (60 ,
61, 65, 71, 75,85,90) Description DR Absent Micro- aneurysm only Mild NPDR Moderate NPDR Moderately Severe NPDR PMod DR – Mild, erate, and Severe Retinal Image Healthy blood vessels with no bulges Small bulges in
blood vessel walls as well as other signs in the retina More changes in the blood vessels in the retina and small spots of blood can become more visible More blood vessels in larger areas of the retina show changes Many of the blood vessels
in the retina show visible changes Incr of n bl eased growth ew, damaged ood vessels DR/DME Endpoint: Diabetic Retinopathy Severity Scale (DRSS)FDA Accepted Endpoint for DR (EYLEA® in PANORAMA Pivotal Trial) EYLEA Product Pamphlet
® Patientsincluded in the ZETA-1 Trial Primary Endpoint of APX3330ZETA-1 TrialPercent of patients with a ≥ 2 step improvement on the DRSS score at week 24 A 13-point Scale Outlining the Various Stages of Diabetic Retinopathy